When the Bunsen burner is lit, what happens to the rod just above it?
Energy is transferred to the metal of the rod just above it. The thermal energy of this part of the rod increases and the rod becomes hot.
|
Previous
Chapter 12: Potential and kinetic energy
|
Next
Chapter 14: Heat insulation and energy saving
|
Chapter overview
2 weeks
In the last chapter we looked at thermal systems which transfer energy. This chapter expands on this and looks at the different ways that thermal energy is transferred between different objects.
It is important to understand the difference between heat, as a concept, and temperature. Temperature is a measure of how hot or cold an object is; it is a measure of the average kinetic energy of the particles of a substance. Heat is the energy transferred between two objects as a consequence of the temperature difference between them. It is also true when energy is transferred between a system and the environment as a consequence of the temperature difference between them. Temperature is measured in degrees Celsius (°C) or degrees Kelvin (K) while heat is measured in joules (J).
3.1 Heating as a transfer of energy (0.5 hours)
3.2 Conduction (2 hours)
|
Tasks |
Skills |
Recommendation |
|
Activity: Conduction through a metal rod |
Experimentation, observation |
Suggested |
|
Investigation: Do all materials conduct heat in the same way? |
Hypothesising, investigating, evaluating |
Suggested |
|
Investigation: Which metals are the best conductors of heat? |
Identifying, hypothesising, observation, writing, recording, drawing graphs. evaluating |
CAPS suggested |
3.3 Convection (2 hours)
|
Tasks |
Skills |
Recommendation |
|
Activity: Convection in water |
Experimenting, observation, comparing |
CAPS suggested |
|
Activity: Does smoke move up or down? |
Observing, explaining |
Suggested |
|
Activity: Where do I put my radiator and air-conditioner? |
Evaluating, drawing, discussing |
CAPS suggested |
3.4 Radiation (1.5 hours)
|
Tasks |
Skills |
Recommendation |
|
Activity: Radiation from a candle |
Observing, examining, explaining |
CAPS suggested |
|
Investigation: Which surfaces absorb the most radiation? |
Measuring, recording, hypothesising, identifying, observing, drawing graphs |
CAPS suggested |
In the last chapter we looked at thermal systems. The thermal energy of an object is the amount of energy it has inside of it, in other words, its internal energy. In a thermal system, thermal energy is transferred from one object to another. Heat is the transfer of thermal energy from a system to its surroundings or from one object to another. This transfer of energy is from the object at a higher temperature to the object at a lower temperature.
It is very important to know that, in science, heat and temperature are not the same thing.
Heat is the transfer of thermal energy from a system to its surroundings or from one object to another as a result of a difference in temperature. Heat is measured in joules (J). This is because heat is a transfer of energy.
Temperature is a measure of how hot or cold a substance feels and it is measured in degrees Celsius (°C). Temperature is a measure of the average kinetic energy of the particles in an object or system. We use a thermometer to measure the temperature of an object or substance.
Complete the following table to summarize the differences between heat and temperature
|
Heat |
Temperature |
|
|
Definition |
||
|
Unit of measurement |
||
|
Symbol for unit |
Here is the completed table:
|
Heat |
Temperature |
|
|
Definition |
The transfer of energy from a hotter object to a colder object, or from a system to its surroundings |
A measure of how hot or cold a substance feels. A measure of the average kinetic energy of the particles of a substance. |
|
Unit of measurement |
Joules |
degrees Celsius |
|
Symbol for unit |
J |
°C |
Heat is the transfer of energy. During energy transfer, the energy moves from the hotter object to the colder object. This means that the hotter object will cool down and the colder object will warm up. The energy transfer will continue until both objects are at the same temperature.
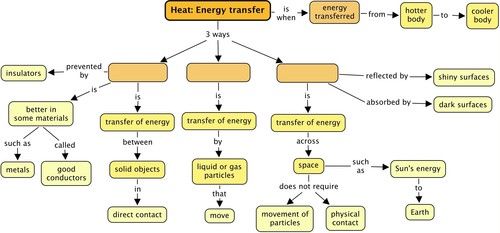
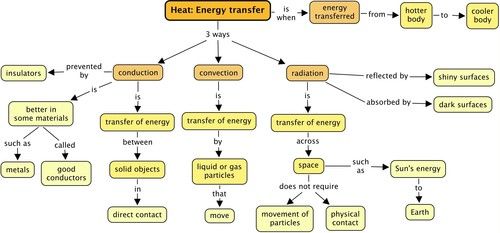
There are 3 ways in which thermal energy can be transferred from one object/substance to another, or from a system to its surroundings:
A rap song to introduce you to (and help you remember!) conduction, convection and radiation.
Let's have a look at these in more detail.
A suggestion to introduce this topic is to ask learners what happens to a metal teaspoon when they put it in their hot beverage. If possible, demonstrate this briefly in class, even with a hot glass of water and a metal rod. In addition, use a plastic teaspoon to demonstrate the difference as plastic is an insulator.
Have you noticed that when you put a cold, metal teaspoon into your hot cup of tea, the teaspoon handle also warms up after a while? Have you ever wondered how this warmth "moved" from the hot tea to the cold teaspoon and warmed it up? This is one way in which energy is transferred and this is called conduction. Let's find out how it works.

When energy is transferred to an object, the energy of the particles increases. This means the particles have more kinetic energy and they start to move and vibrate faster. As the particles are moving faster they "bump" into other particles and transfer some of their energy to those neighbouring particles. In this way, the energy is transferred through the substance to the other end. This process is called conduction. The particles conduct the energy through the substance, as shown in the diagram.
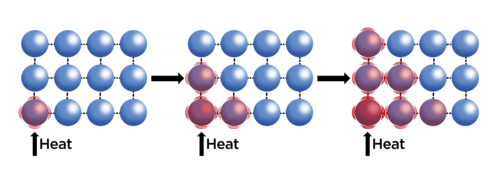
Let's demonstrate this practically.
Set this demonstration up in front of the class as you start to talk about conduction.
MATERIALS:
INSTRUCTIONS:
INSTRUCTIONS:
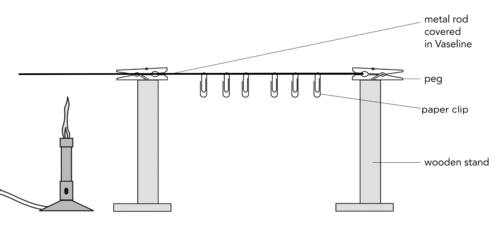
As an extension exercise you could include another investigation in which you measure the rate of energy conduction along a metal rod. Repeat the experiment placing drawing pins at 5cm intervals on a long metal rod. Clamp the metal rod and heat one end over a Bunsen burner. Use a stopwatch to time how long it takes for each drawing pin to drop and record the results on a graph. This could be further extended by using different metals and putting all the results on a single set of axes. The gradient of the graphs would give the rate of heat conduction.
QUESTIONS:
When the Bunsen burner is lit, what happens to the rod just above it?
Energy is transferred to the metal of the rod just above it. The thermal energy of this part of the rod increases and the rod becomes hot.
Which pin or paperclip dropped off the metal rod first? The one closest to or furthest from the Bunsen burner?
The one closest to the Bunsen burner dropped off first.
What does this tell us about the way in which heat is conducted along the rod?
The heat is transferred from where it is hottest to the colder end of the rod.
Let's think about the teaspoon in the tea again. The tea is hot and the metal spoon is cold. When you put the metal teaspoon into the hot tea some of the thermal energy from the tea is transferred to the metal particles. The metal particles start to vibrate faster and collide with their neighbouring particles. These collisions spread the thermal energy up through the teaspoon. This makes the handle of the teaspoon feel hot.
Conduction is the transfer of thermal energy between objects that are touching. In the teaspoon example, the particles of the tea are touching the particles of the metal spoon, which in turn are touching each other, and this is how heat is conducted from one object to the other.
Do all materials conduct heat in the same way? Let's find out.
Misconceptions about temperature. Why do you think your carpet feels warmer than tiles in winter? Watch this video to find out.
In response to the video in the margin box about why your carpet feels warmer than the tiles in winter, you can come back to this question after you have performed the following investigation, and also looked at the example of the cake tin and the cake straight out of the oven. You can lead the discussion in the following way:
This investigation will show the learners that metals conduct heat better than non-metals. If possible, watch the Veritasium video provided in the visit link before class about the misconceptions surrounding temperature and which demonstrates this activity. Start off by asking learners to feel the blocks and ask which one feels colder. The aluminium block will feel colder. Then ask them which block they think will melt the ice cube the fastest. as in the video, most people think that the ice cube will melt faster on the plastic block as it feels warmer than the aluminium block. However, this is a misconception, and will be demonstrated in the activity that it is in fact the aluminium block which causes the ice cube to melt faster as metals are a better conductors of heat.
AIM: To investigate which materials are the best conductors of heat.
In this investigation, we will be placing an ice cube on a plastic block and on an aluminium block and observing which ice cube melts the fastest.
HYPOTHESIS: Write a hypothesis for this investigation. Which block do you think will melt the ice cube the fastest?
Learners might hypothesize that the ice cube will melt faster on the plastic than the aluminium block. If they do, make sure that they come back to reject their hypothesis and revise it.
MATERIALS AND APPARATUS:
You can use any piece of plastic and aluminium (or other metal) that you can find. if possible, use a circular ring to stop the melted water from spilling.
METHOD:
First feel the plastic block and the aluminium block. Describe how they feel.
Learners will note that the plastic block feels warmer than the metal block.
OBSERVATIONS:
Which ice cube starts to melt first and the fastest?
The ice cube on the aluminium/metal block melts first.
Is this what you thought would happen? Refer back to your hypothesis.
Learner-dependent answer. Most people generally have the misconception that the ice cube will melt faster on the plastic block, rather than the metal block.
CONCLUSIONS:
Metal is a better conductor of heat than plastic as the ice cube on the metal melted first.
We will discuss this in the next paragraph about why this happens.
So how does this work? This is to do with thermal conductivity, the rate at which heat is conducted from one object to another.
When you originally felt the blocks, you felt that the plastic block was warmer. But, what we observed is that the aluminium or metal block melted the ice cube faster. This is because the metal block is conducting the heat faster to the ice cube. The plastic block is a worse thermal conductor so less heat is being transferred to the ice cube and so it does not melt as fast.
Why then does the aluminium block feel colder than the plastic block?
This is because the aluminium conducts heat faster away from your hand than the plastic does. Therefore the aluminium block feels colder and the plastic block feels warmer. When you touch something, you do not actually feel the temperature. Rather you feel the rate at which heat is either conducted away from or towards you.
Let's think of another example of baking a cake. Imagine you have just finished baking a cake in the oven at 180 °C.

When you remove the cake from the oven, which is more likely to burn you more, the metal cake tin, or the cake?
The most likely answer is that the cake tin will give you a more serious burn.
For the next question, get learners to speculate about what they think about the temperature of the cake tin and the actual tin. Many people have the misconception that the tin is hotter than the cake as it feels hotter. They are actually at the same temperature as they have both been baking at 180 °C.
Do you think the cake and the tin are at the same temperature when you remove them from the oven? Why?
Yes, the cake and the tin are both at the same temperature as they have been baking at 180 oC. Learners might be inclined to say that the tin is at a higher temperature than the cake as it feels hotter and the metal tin will give you a more serious burn than the actual cake. This is a misconception and you must discuss this. As with the example of the aluminium and plastic block, the cake tin and the cake are at the same temperature. But, the metal tin conducts heat faster towards your hand than the cake does. Therefore, the metal tin will feel hotter and is more likely to give you a serious burn than the cake does. When you touch something, you do not actually feel the temperature. Rather you feel the rate at which heat is either conducted away from or towards you.
Misconceptions about heat: Why is a cake tin more likely to burn you than the actual cake?https://www.youtube.com/watch?v=hNGJ0WHXMyE
If you have the opportunity, watch the video in theVisit margin box by typing the link into your internet browser, even on your mobile phone. This video demonstrates the cake and cake tin example.
What we have seen here is another example of thermal conductivity. The tin will conduct heat much faster to your hand than the cake, so the tin will burn you, but the cake will not. The tin and the cake are at the same temperature.
So what have we learnt? Metals conduct heat better than non-metals.
There are substances that allow thermal energy to be conducted through them and so they are called conductors.
There are substances that do not allow thermal energy to be conducted through them and so they are called insulators.
This links back to what we learned in Matter and Materials about the properties of materials and how their properties determine their uses. Remind learners of the activities they did in Matter and Materials, especially linked to conductivity.
Remember, just because a materialfeels colder, does not mean it has a lower temperature. It may just be that it is conducting heat faster away from your hand.
Now that we know that metals are good conductors of heat, do you think all metals conduct heat equally well? Let's investigate which metals are better conductors.
We are going to see which metal is the better conductor of thermal energy. To do this we will see which metal becomes hot first.
Make sure you know how to use a Bunsen burner safely.
Now that we have established that metals conduct heat energy better than non-metals, the learners will investigate which metals are the best conductors of heat. This investigation requires more heat than the previous one and so the learners should not test the conduction with their fingers.
Spend a few minutes before the learners begin by demonstrating the correct procedure for lighting a Bunsen burner. There are many different instructional videos on the internet, such as the one identified in the visit box in the margin. Here are a list of instructions for your reference:
You can ask the learners to draw posters explaining how to light a Bunsen burner as an additional exercise if you feel they need the extra practice and reminders.
Remember that the tripods and metal rods that the learners use will get quite hot during this experiment. Make sure to allow the apparatus to cool before packing it away.
AIM: To identify whether some metals are better conductors of heat than other metals.
IDENTIFY VARIABLES :
Read through the method and look carefully at the diagram for the investigation to identify the different variables required.
Which variable are you going to change?
Material being tested i.e. iron, copper, brass or aluminium
What do we call the variable that you are going to change?
This would be the independent variable
Which variable are you going to measure?
Time taken for the drawing pin to drop.
What do we call the variable that you are going to measure?
The dependent variable
Which variables must be kept the same?
Length and thickness of the material should be the same for each material used. Distance of the drawing pin from the heat source.
What do we call the variables which must be kept the same?
Constants
HYPOTHESIS:
Write a hypothesis for this investigation.
Learner-dependent answer. Learners can hypothesise about which metal they think will be the best conductor, for example, the copper rod will be the best conductor.
MATERIALS AND APPARATUS:
The materials listed here are a suggestion. You can use alternative apparatus to still do this investigation. For example, a spirit burner could also be used to heat the rods. If you do not have a tripod stand, you can place the metal rods on another stand, such as a block of wood, with the ends sticking out one side to still reach over the Bunsen burner. Paper clips can also be used instead of drawing pins. The type of metals are not important as long as you have different metals of the same length.
METHOD:
The cardboard is an insulator and will stop the heat from the rods transferring to the tripod itself. The loss of heat from the rods could affect the results.
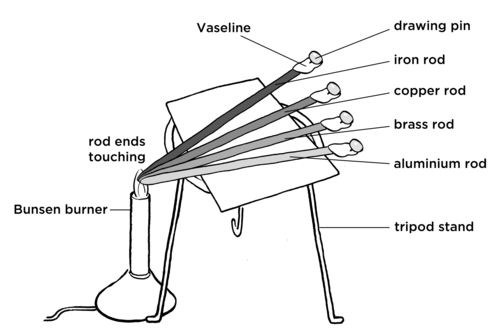
RESULTS AND OBSERVATIONS:
Record your results in the following table.
|
Type of metal |
Time taken for pin to drop off (seconds) |
|
iron |
|
|
copper |
|
|
brass |
|
|
aluminium |
Now draw a bar graph to show your results. Do not forget to give your graph a heading to describe what it represents.
Which variable should be on the horizontal x-axis?
The type of material should be on the horizontal axis. This is the independent variable.
Which variable should be on the vertical axis?
The time taken for the drawing pin to to fall off should be on the vertical axis. This is the dependent variable.
Why do you think that a bar graph is suitable for this investigation?
The independent variable/type of material is not a number value and so it does not need a number line. A bar graph is used to represent non-number or non-continuous data.
The independent variable is always drawn on the x-axis with the dependent variable on the y-axis. Both axes must be labelled and show the units of measurement. The graph should have a heading.
An example set of data is given here with the accompanying bar graph as a reference. Your results may vary from these presented here.
|
Type of metal |
Time taken for pin to drop off (seconds) |
|
iron |
60 |
|
copper |
30 |
|
brass |
50 |
|
aluminium |
40 |
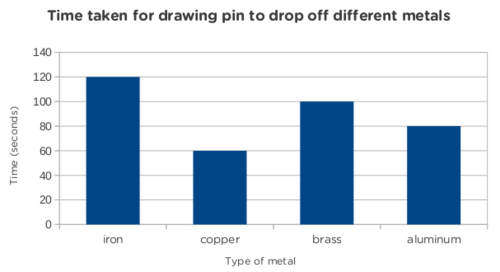
ANALYSIS :
Which bar on your graph is the longest?
The longest bar should be the iron.
Which bar is the shortest?
The shortest bar should be the copper.
Write down the materials in order of how fast they conducted heat from the quickest to the slowest.
Activity-dependent answer.
Why does the Vaseline melt?
The heat is transferred by conduction through the metal rod and to the Vaseline causing an increase in its temperature and then a change of state (solid to liquid).
Why do you think it was necessary to place the piece of cardboard or paper on the tripod stand underneath the metal rods. Hint: The tripod stand is also made of metal.
The cardboard acts as an insulator to prevent heat from transferring to the stand from the rods. For the purpose of this experiment, the heat should transfer down to the different metal rods only.
Why do you think it is necessary to use the same amount of Vaseline on the ends of each rod?
This is so that the test is fair, otherwise some drawing pins might be stuck on better than others, leading to inaccurate results.
Do you think we could have performed this investigation if our rods were of different lengths? Why?
No, otherwise it would not be a fair test as the heat will have to be conducted further in some rods than in others, leading to inaccurate results.
EVALUATION:
It is always important to evaluate our investigations to see if there is anything we would change or improve on.
Is there anything that went wrong in your investigation that you could have prevented?
Learner-dependent answer.
If you were to repeat this investigation, what would you change?
Learner-dependent answer. Examples include: repeating the same experiment three times and averaging the results, increasing the number of metals tested.
CONCLUSIONS:
This answer will depend on their experimental results, and the exact metals which you used in the investigation.
In this section we looked at how heat is conducted through metal rods and other objects. These were all solid objects. How is energy transferred through liquids or gases? Let's find out in the next section.
As an introduction to this section, you can simulate the "sitting in a bath" concept by filling a rectangular plastic tub or small water tank with cold water and then pouring hot water into one side. Invite the learners to feel the cold side of the tub and then feel it a few minutes later.
If you can get hold of a lava lamp, this can make a very exciting introduction to the lesson. You can turn the lights off and place the lava lamp on your desk for when learners come into the class. You can then explain that you are going to find out why the blobs rise and then fall back down in the lava lamp. If you do not have a lava lamp, you can also play this video:
Think of a pot of water on a stove. Only the bottom of the pot touches the stove plate, but all of the water inside the pot, even the water not touching the sides, becomes warmer. How does the energy transfer throughout the water in the pot? The transfer of energy is because of convection.
Let's do an activity that will help us to visualise how convection occurs.
Colourful convection currents (video)
MATERIALS:
Take note that you only need a few grains of potassium permanganate, otherwise you will not see anything.
An alternative to the above materials is the following:
INSTRUCTIONS:
Learners must not just throw the potassium permanganate into the water. It is important that they place it carefully in one side of the bottom of the beaker so that they can see how the currents in the water move.
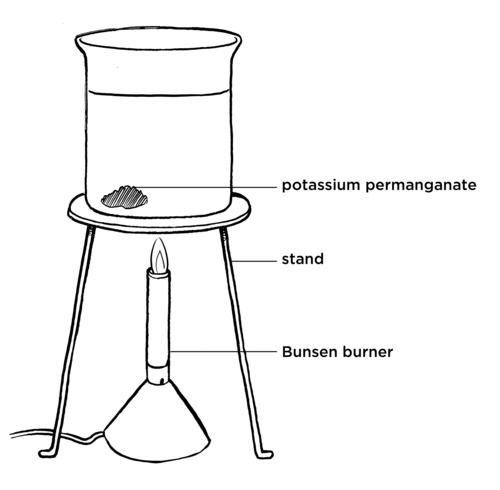
QUESTIONS:
What did you see as the water started to warm up in the beaker that was heated? Draw a picture to show what you see.
Learners should see the purple from the dissolved potassium permanganate moving in a circle upwards through the water.
What is happening to the potassium permanganate in this beaker?
As the potassium permanganate dissolves in the water it is being dragged through the water.
Can you explain the pattern you saw?
The warm water is rising and being replaced by cooler water.
NOTE:
At this point the learners are not aware of the theory behind convection currents and so their answers will be quite simple.
Compare this to the beaker which was not heated. What did you observe in this beaker?
The potassium permanganate will dissolve, but it will not form rising currents. It will diffuse evenly and densely at the bottom of the beaker. Over a long time it will spread out evenly throughout the water.
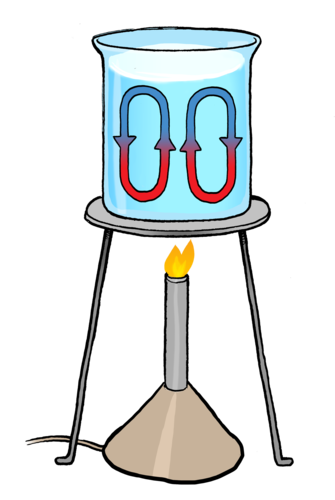
Let's now explain what we observed in the last activity. Convection is the transfer of thermal energy from one place to another by the movement of gas or liquid particles. How does this happen?
As a gas or liquid is heated, the substance expands. This is because the particles in liquids and gases gain kinetic energy when they are heated and start to move faster. They therefore take up more space as the particles move further apart. This causes the heated liquid or gas to move upwards and the colder liquid or gas moves downwards. When the warm liquid or gas reaches the top it cools down again and therefore moves back down again.
We then say that the heated liquid or gas is less dense as the same particles are now taking up a larger space. We will learn more about density next year in Gr 8.
In the last activity, the water particles gained kinetic energy and moved apart from each other, therefore taking up more space. This water then moves upwards as it is less dense than the cold water, meaning it it lighter than the cold water. We were able to observe this as the potassium permanganate dissolved in the water and moved with the water particles, and then moved downwards again as the water cooled.
This movement of liquid or gas, is called a convection current, and energy is transferred from one area in the liquid or gas to another. Have a look at the diagram which shows a convection current.
The learners need to be careful with this experiment. It is easy to set the T-shaped cardboard alight with the candle and they should be careful not to burn their fingers when lighting the candles as well.
MATERIALS:
INSTRUCTIONS:
You can drip some wax onto the base and then stick the candle onto this to make it stand.
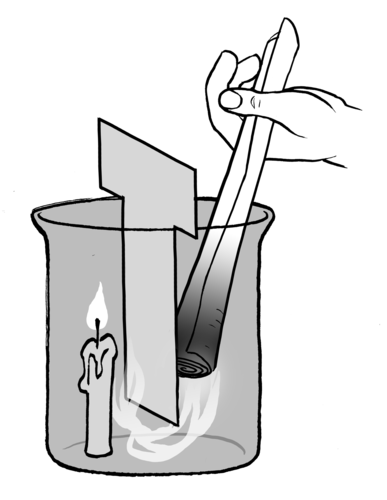
QUESTIONS:
What happens to the smoke from the paper?
The smoke is drawn down under the cardboard and up next to the candle.
NOTE:
Some of the smoke particles may move upwards.
Why do you think the smoke moves in this way?
The candle heats the air above it which creates a convection current which draws the cooler air on the other side of the cardboard towards the candle. This movement of the air particles pulls the smoke particles with it. The smoke particles allow us to visualise the convection current.
In the last two activities, we have observed convection currents in a liquid and in a gas. Convection currents can only form in gases and liquids as these particles are free to move around. They are not held in fixed positions like in a solid. Solid particles are held together too tightly for them to move when heated. Solid particles will only vibrate faster when heated but will not move from their positions.
The solid particles will only move from their positions when they have gained enough kinetic energy for a change of state to occur and the solid melts to become a liquid.
The blobs in a lava lamp move up and down in the lamp as they first heat and expand and then reach the surface and cool so they move back down again.

How does a lava lamp work? (video)
Now that we have learned about convection, how can we apply this in the world around us? It is interesting to learn about concepts and theories in science, but it is even more interesting when we discover how this has an influence in our daily lives.
Imagine that your teacher has been given a heater and an air-conditioning unit for your classroom. The heater will warm your classroom in winter and the air-conditioner will keep you cool in summer. You need to help you teacher decide where each item should go in the classroom. Should they go on the wall near the ceiling or near the floor? Should they go next to a window?

INSTRUCTIONS:
Discuss where in your classroom you would place a heater so that it can effectively heat up the room. Draw a diagram to explain your choice.
A heater should be placed near the floor. As it heats the air around it, the warm air will rise and be replaced by cool air. The cool air is then warmed and rises. This creates a convection current which will warm the entire room. The diagram should show the upward circulation of the warm air.
Discuss where in your classroom you would install the air-conditioner so that it can effectively cool the room. Draw a diagram to explain your choice.
An air-conditioner should be placed near the ceiling. As it cools the warm air near the ceiling the cool air moves downward towards the floor and is replaced by warm air from below. The warm air is then cooled by the air-conditioner. This creates a convection current which will cool the entire room. The diagram should show the downward circulation of the cool air.
Try to find an air-conditioner or heating specialist who you can interview. Ask them to explain the best way to install the air-conditioner and a heater.
We have now looked at how energy is transferred through different materials, whether they are solids (conduction) or liquids and gases (convection). But, what about if there are no particles to transfer the thermal energy? Is there still a way for energy to be transferred?
Have you ever wondered how the Sun is able to warm us even though it is so far away? The energy is transferred from the Sun to everything on the Earth. The Sun does not need to be touching the Earth for the energy to be transferred. Also, there is space in between the Earth and the Sun. The energy from the Sun is able to warm us without the Sun ever touching us.
This transfer of energy is called radiation. It is different to conduction or convection as it does not require objects to be touching each other or the movement of particles.
Radiation comes from the Greek wordradius, meaning a beam of light.
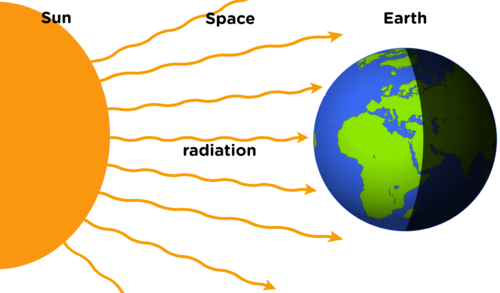
It takes light about 8 minutes to travel from the Sun to Earth.
We can also see how heat is transferred by radiation here on Earth, and not just between the Sun and the Earth. Let's demonstrate the difference between radiation and convection using a candle.
A suggestion is to do this as a demonstration and get learners to come up in small groups. You can then control how close they put their hands to the flame. Take note that heat radiates in all directions around the source of thermal energy (including the top of the candle). What makes us feel the heat more at the top is the effect of convection currents of the hot air moving up. They should first hold their hands above the flame to feel the heat from convection. Then they should hold their hands next to it to feel the heat transfer from radiation. Finally, you can also demonstrate conduction using a metal spoon and holding it in the flame.
MATERIALS:
INSTRUCTIONS:
QUESTIONS:
We know now that heat from a candle will be transferred to the air around it. These will warm up. Where will this air move to?
The air particles will move upwards.
What is this called?
Convection.
So, when you hold your hand above the candle, what do you feel and why?
When you hold your hand above the candle, the warm air particles transfer the energy to your hand causing your hand to warm up and you feel the increase in temperature.
But, what about when you hold your hand on the side of the candle? Could you also feel warmth from the candle?
Yes
This is not convection as the air particles do not travel sideways when they warm up from the flame. So, how is energy transferred to your hand when you feel the warmth on the side of the candle?
The energy is transferred by radiation.
Lastly, if your teacher placed a metal spoon in the candle flame and you felt the end, how would it feel after a little while?
It would also feel warm.
How was the energy transferred from the flame to the end of the spoon?
The energy was transferred by conduction.
This photo shows all three forms of how heat is transferred. Explain which type of heat transfer is represented by each hand.

The hand on the right holding the spoon represents conduction as the heat is transferred from the flame through the metal of the spoon. The hand above the candle represents convection as heat is transferred from the flame by moving air particles which warm up and rise. The hand above the candle will also experience heat from radiation as heat is radiated in all directions. The hand on the left next to the candle represents radiation as energy is transferred from the source through space to the hand.
As we saw in the last activity, energy is transferred from the candle to your hand by convection and by radiation. Have you ever stood next to a huge fire? You will feel the radiating heat even though the air might be very cold. This is because the energy is transferred to you by radiation through the spaces between the particles in air.
What about if you touch a black wall or a white wall? Do you think there is a difference in how different surfaces absorb and reflect radiation? Let's find out by doing an investigation.
This investigation looks at the way different materials absorb radiation or reflect it. It is important that the surface area of each material is kept the same so that the results are reliable. This investigation will work best on a hot, sunny day. Try to find the sunniest place you can on the school grounds in order to conduct this investigation.
We are going to investigate which surfaces absorb the most heat, using dark coloured paper, light coloured paper and shiny paper, such as aluminium foil. We will use the temperature inside an envelope made from each kind of paper as a measure of the amount of heat the paper absorbed. Why do you think we can do this?
Discuss this with your class as it is important that the understand why they are doing the investigation. When the paper envelope absorbs heat, the energy will then be transferred to the air inside the envelopes. This will then cause a rise in temperature which the thermometer will show. The more energy that is absorbed, the more that is transferred to the interior, and the higher the temperature. The paper that reflects the most energy will show the smallest increase in temperature.
INVESTIGATIVE QUESTION:
Which surfaces will absorb the most radiation from the Sun and therefore increase in temperature the fastest?
VARIABLES
Which variable are you going to measure?
The temperature of the substance.
What do we call the variable you have measured?
The dependent variable.
Which variable are you going to change?
The type of material.
What do we call this variable?
Independent variable.
What must be kept the same for all the different materials?
The surface area of each substance which is exposed to the Sun must be the same (ie. the size of the envelope). The length of time that the materials are exposed to the Sun.
HYPOTHESIS:
Write a hypothesis for this investigation.
Learner-dependent answer. The hypothesis could be: 'The shiny surface will absorb the least heat, and the black/dark coloured paper will absorb the most.'
MATERIALS AND APPARATUS:
You can also extend the investigation by testing more colours, such as red and yellow to see how they compare.
METHOD:
RESULTS AND OBSERVATIONS:
The results for this experiment are dependant on the size of the paper envelope that the learners make as well as the amount of sunlight falling on the envelopes. The readings may also fluctuate from time to time as a result of cloud covering.
Record your results in the following table.
|
Time (minutes) |
Temperature in black paper envelope (°C) |
Temperature in white paper envelope (°C) |
Temperature in aluminium foil envelope (°C) |
|
0 |
|||
|
2 |
|||
|
4 |
|||
|
6 |
|||
|
8 |
|||
|
10 |
|||
|
12 |
|||
|
14 |
|||
|
16 |
Draw a line graph for each of the envelopes in the space below. Do not forget to give your graph a heading.
Time should be plotted on the horizontal axis with temperature on the vertical axis. Draw three different graphs for the three different materials. Comparing the slopes of the three graphs will allow the learners to determine which material warmed up fastest. The line with the steepest slope heated the fastest.
The black paper should increase in temperature the fastest and so it would have the steepest curve. The aluminium envelope should increase in temperature the slowest and have the shallowest curve, with the white paper in between.
The graph should have a title. An example of a suitable title would be 'A comparison of the rate of temperature increase of different surfaces.'
ANALYSIS:
What do you notice about the shapes of the graphs you drew? Are the graphs straight lines or curves?
Activity-dependent answer. The values obtained will depend on the size of the envelopes the learners make as well as the amount of sunlight to which the envelopes were exposed. It is important that they should see an increasing trend in the lines of the graph.
Which line on your graph is the steepest? What does this tell us?
The graph representing the black paper should be the steepest graph. This means this envelope increased in temperature the fastest. This is because the black, matt colour absorbs the most radiation.
Compare your results for the white paper and the shiny surface. What does this tell you.
The envelope made out of aluminium foil should show the smallest increase in temperature as shiny surfaces reflect heat.
EVALUATION:
Did the investigation run smoothly? Or is there anything you would change?
Learner-dependent answer. Learners should discuss the quality of their method and whether they got the results that they expected to get. They could suggest repeating the experiment three times and getting an average increase over time.
Did you get any results which did not seem to fit the overall pattern?
Learner-dependent answer. Some learners may get outliers but others may have clear results with a clear patterns.
CONCLUSION:
Write a conclusion for your investigation. Remember to refer back to the investigative question that we wanted to answer.
Learners should conclude that black surfaces absorb the most radiation and therefore show the biggest and fastest increase in temperature, whereas shiny surfaces absorb the least, as they reflect the most.
Radiation from the Sun is essential to life on Earth, but ultraviolet radiation from the Sun can also be very damaging our skin. Remember to wear suncream and a hat when outside and avoid being in direct sunlight between 11am and 2pm.
The investigation showed that the dark envelope showed the biggest increase in temperature. The lighter coloured envelope showed a smaller increase in temperature. The envelope made out of a shiny material showed the smallest increase in temperature.
So what have we learnt? Dark colours seem to absorb more of the Sun's radiation than light or reflective colours. So, if you want to stay warm on a cold day, dark clothing will absorb more of the available warmth from the Sun's radiation than light colours.
The average summer temperature in Hotazel, a town in the Northern Cape is about 34 °C. If you lived in Hotazel and needed to buy a new car, would you buy a light or dark-coloured car? Explain why.
The best colour to buy would be a white car because, as seen in the investigation, light colours absorb less heat than dark colours. So a light-coloured car will ideally remain the coolest on the inside.
You have the option of getting the car sprayed to make the surface more shiny. Do you think this will help keep the car cool in hot, summer months? Explain why.
Yes, it will help, as shiny surfaces are more reflective and so more radiant heat is reflected rather than absorbed, keeping the inside of the car cooler.
Concept map
Below is a concept map showing how the different topics about heat together. You need to fill in the three different ways that energy can be transferred, as discussed in this chapter, but you cannot just put anyone into any box. You need to study the concepts which come after and explain each way of transferring energy during heating.
How is energy being transferred in the following photos showing different heating processes? Write down conduction, convection or radiation. Some illustrations may show more than one form. [4 marks]
 The heat from the Sun travels to Earth. |
 Cooking food on a braai or fire. |
 Boiling water in a metal pot. |
 A heater in a room. |
 The heat from the Sun travels to Earth. |
 Cooking food on a braai or fire. |
|
Radiation |
Convection (and also some radiation) |
 Boiling water in a metal pot. |
 A heater in a room. |
|
Conduction (through the metal) and convection (in the water) |
Radiation and convection |
In each of the following situations, identify the method of energy transfer taking place (conduction, convection, radiation).
A fireplace has a glass screen in front of it. The person sitting in a chair next to the fireplace chair feels hot due to ________. [1 mark]
When you stir your tea with a metal spoon the handle gets hot because of ________. [1 mark]
When you are lying on the beach your skin feels hot because of _______. [1 mark]
radiation
conduction
radiation
Draw energy transfer flow charts for the following: You buy a cup of hot chocolate and hold it in your hands on a cold winter day. [2 marks]
The energy is transferred from the cup to the hands by conduction.
NOTE:
One of the marks is for choosing the correct direction of the energy transfer. The second mark is for drawing it in the form of a flow chart.
Your parents have a metal hot water geyser and they are complaining about the amount of energy needed to keep the water hot. What can you recommend your parents could do to prevent energy loss from the geyser? Explain your answer. [4 marks]
Metals are good conductors of heat and so the heat from the water is transferred out of the geyser. A (shiny foil) insulating blanket could be used to wrap around the geyser. The air between the blanket and the geyser is a poor conductor of heat so the heat loss will be slower.
Explain why the heating element for a kettle is at the bottom and not at the top. [3 marks]
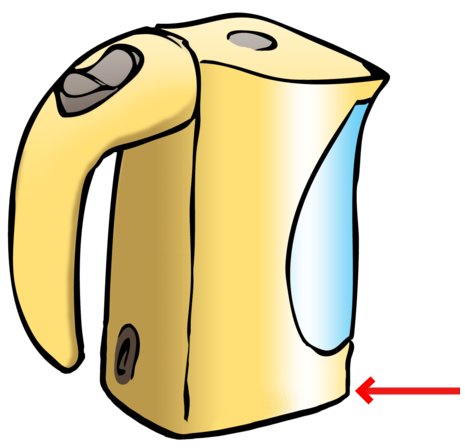
The heating element is at the bottom because as the element transfers energy to the water, the water expands and moves upwards and the colder water (slower moving particles) will sink to the bottom, forming a convection current This cycle will ensure that all the water is heated as quickly as possible. If the element was at the top, the water at the bottom would take much longer to boil.
NOTE:
Learners must mention the term convection current.
Explain why you think the water boils throughout the kettle pot and not just at the bottom? [2 marks]
The water at the bottom of the pot gets hot and then moves to the top of the pot because of convection. This allows the cold water to sink to the bottom and heat up. This constant circulation allows all of the water to heat up and boil.
Explain why you think take-away coffee is sold in styrofoam cups rather than ceramic cups. [2 marks]
Normal ceramic cups are good conductors of heat and so the energy from the coffee is transferred quickly through the cup to the surroundings. The styrofoam is a poor conductor of heat and so it does not allow the energy from the coffee to move quickly to the surrounding air, so the coffee stays warmer for longer.
Explain why you think two thin blankets can sometimes be warmer than one thick blanket. [2 marks]
Air is trapped between the two blankets. The air is a very poor conductor of heat and so it becomes an extra insulating layer which slows down the loss of energy from your body. One blanket cannot trap as much air and so isn't as warm as two blankets.
Explain why birds fluff up their feathers to stay warm, especially in winter. [2 marks]
Birds fluff up their feathers so that more air gets trapped between the feathers. The air is a poor conductor of heat and so the energy from the birds body is not transferred to the surroundings.
Why should you place an air conditioner at the top of a room, near the ceiling, rather than at the bottom near the floor? [2 marks]
This is because cold air will move downwards, therefore cooling the room, and the hot air will rise and can therefore be removed by the air conditioner at the top of the room, near the ceiling.
Imagine you want to build a small enclosure for some chickens on your property. You have an outside area for them that is made from barbed wire, and you have made a small inside, covered enclosure for them out of bricks and cement which you would like to paint. You know that it can get quite cold in winter in your area so you want the house to be as warm as possible for the chickens. What colour paint are you going to choose to paint the outside of chicken house? Will it be a dark-coloured paint, such as brown or black, or a light-coloured paint, such as white or yellow? Explain your choice. [4 marks]
The best choice to keep the house as warm as possible on the inside is a dark-coloured paint. This is because the dark colours absorb more radiant heat from the Sun during the day, than the light colours, which reflect heat. The dark paint will absorb the heat and it will be transferred to the air inside of the house, making it warmer, especially during winter.
Total [30 marks]
|
Previous
Chapter 12: Potential and kinetic energy
|
Table of Contents |
Next
Chapter 14: Heat insulation and energy saving
|