H2
Reactions of acids with metals
Chapter overview
0.5 week
This is a short chapter to conclude the series of reactions that learners will have been exposed to this term. The last reactions to look at are those between an acid and a metal. At the end of this chapter, there is a short activity on some of the careers in the chemical industry. Although this is not for assessment purposes, if you do not have time to do it in class, we encourage you to encourage or get your learners to do it as a homework activity. Seeing the real world application for what they learn in the classroom is a very important part of the learning process and in discovering what is possible through science and technology.
7.1 The reaction of an acid with a metal (1.5 hours)
|
Tasks |
Skills |
Recommendation |
|
Activity: Testing for hydrogen gas |
Remembering, balancing chemical equations |
Optional |
|
Investigation: The reaction between magnesium and hydrochloric acid |
Hypothesising, preparing, measuring, observing, interpreting |
CAPS suggested |
|
Activity: Writing the chemical equation |
Writing and balancing chemical equations |
Optional (Suggested) |
|
Activity: Other careers in chemistry |
Researching, comparing, describing |
Optional |
- What do we get when a metal reacts with an acid?
- What is the general equation for the reaction between a metal and an acid?
- How do we write the word equation and the balanced chemical equation?
- How can we test for the presence of hydrogen gas?
The reaction of an acid with a metal
- diatomic
- density
- characteristic
- presence
- chemist
- pharmacist
In the previous chapter we learnt about the reactions of acids with a variety of bases: metal oxides, metal hydroxides and metal carbonates. We learnt how to write general equations, word equations and chemical equations for those reactions.
In this chapter we will investigate one final type of reaction, namely the reaction between an acid and a metal.
First, we will do an investigation to observe the reaction and then we will write equations to represent it. Before we do this, however, we have to take a quick detour to learn something interesting about hydrogen gas.
Testing for hydrogen gas
This activity introduces the test for hydrogen gas. It is optional, however, the test will be sued in the following investigation so if you do not do this activity in class, a suggestion is for learners to do it in their own time, or to just explain the hydrogen test briefly before proceeding to the investigation.
Hydrogen gas is a diatomic gas. What does this mean?
That means each molecule of hydrogen gas consists of two H atoms.
Hydrogen can be found in the top left hand corner of the periodic table.
The position of hydrogen in the periodic table tells us that it is the lightest of all the elements. It has the smallest atomic mass. Because the element hydrogen is a gas (even though it is a diatomic one), it has one of the lowest densities of any substance. Can you remember what density means? Write your own definition below.
Density is the mass of a substance in a given space (volume).
When hydrogen gas is released in a reaction it will immediately rise up, because hydrogen is less dense than air. If you filled a balloon with hydrogen, it would float up and you would need to tie a string to it to prevent it from floating away!

Another interesting thing about hydrogen is that it reacts explosively with oxygen if you bring a flame near it. You may remember learning about this in Chapter 4 about the reactions of non-metals with oxygen. The reaction between a large quantity of hydrogen and oxygen in the air produces a beautiful orange fireball and a very loud boom! Do you remember seeing the following diagram?
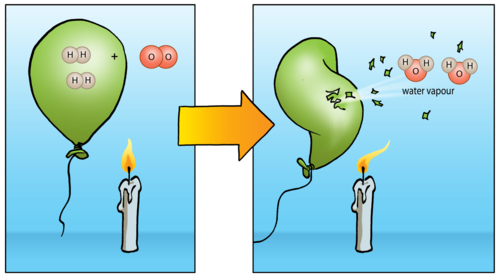
Write the balanced equation for the reaction between hydrogen gas and oxygen below.
2H2 + O2→ 2H2O
The reaction between a tiny amount of hydrogen and oxygen in the air produces a characteristic 'pop' sound and this serves as test for the presence of hydrogen. You can watch the short video clip in the visit box in the margin to see this 'pop'.
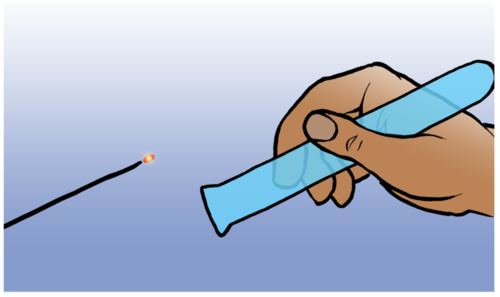
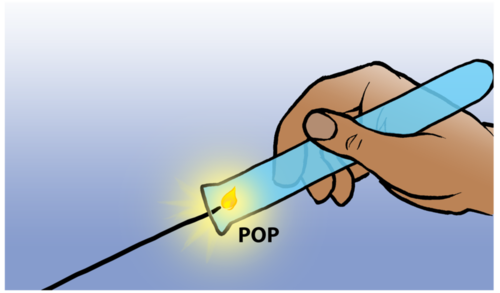
Testing for hydrogen gas
The name hydrogen comes from the Greek words 'hydro' and 'gen' which means 'water generator'
Let's now investigate the reaction between an acid and a metal. You should listen carefully for this 'pop' sound during the investigation. If you hear it, it will signal the presence of hydrogen gas!
The reaction between magnesium and hydrochloric acid
It is recommended that you demonstrate this reaction to learners. There are many ways to perform this demonstration and if you have a tried and trusted method, you should use it by all means. For example, a simple method is to place diluted HCl in a test tube, add a piece of magnesium and then bring a glowing splint to the neck of the test tube so that it goes 'pop' in the presence of the hydrogen gas that is produced. The method we have included here does not require anything too complicated and it has the added fun aspect of blowing hydrogen bubbles and popping them with a candle flame.
The purpose of this investigation is to:
- observe the reaction between hydrochloric acid and magnesium; and
- identify the gaseous product of the reaction between hydrochloric acid and magnesium.
Your teacher will demonstrate the reaction between magnesium and hydrochloric acid, while you make observations. Remember to watch carefully and take detailed notes.
INVESTIGATIVE QUESTION:
One possible question would be: What products will form when magnesium reacts with hydrochloric acid?
HYPOTHESIS:
Some ideas:
- When magnesium reacts with hydrochloric acid, a gas is released.
- When magnesium reacts with hydrochloric acid, hydrogen gas is released.
MATERIALS:
- magnesium ribbon (cut into smallish pieces)
- hydrochloric acid (HCl) solution (1 M)
- large test tube
- retort stand with clamp
- rubber stopper with short length of glass tubing pushed through it
- silicone or rubber tubing (or a glass delivery tube as shown in the set-up below)
- shallow dish filled with soapy water (made by mixing a few teaspoons of dishwashing liquid with water)
To prepare dilute hydrochloric acid solution, slowly and carefully add approximately 100 ml concentrated hydrochloric acid (33% or 11 M) to 900 ml of cold tap water. It is recommended that you wear safety goggles and protective gloves during this step and that you rinse away any acid spills with cold tap water. Since this is just a qualitative experiment, it is not necessary to use distilled water for the solution. It is also not required that you measure the volumes with extreme accuracy. Be careful when handling this solution; even though it is dilute it can still cause burns.
The quantities for this experiment are as follows: 1 g of magnesium will require approximately 42 ml of 1M hydrochloric acid to be consumed. Just more than 900 ml of hydrogen gas will be produced by these quantities of reactants.
METHOD:
The pH of the 1 M HCl solution will be below 1. Since this solution is still very corrosive, it is recommended that you choose one responsible learner to perform the pH measurement on behalf of the class.
- Set up the experiment as shown in the following diagram. Ensure that the end of the delivery tube is below the surface of the soap solution in the dish.
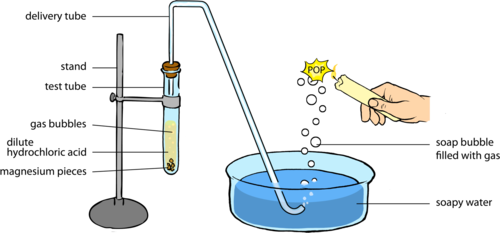
A possible extension is to hold a cold piece of metal or glass above the place where you burst the bubbles so that the water vapour that forms during the reaction condenses on the metal or glass.
- Place approximately 1 g of the magnesium pieces in the test tube, but do not add the hydrochloric acid until everything else is ready to be assembled.
- Add approximately 40 ml of hydrochloric acid and immediately place the stopper on the test tube. The first few bubbles of gas that are released from the end of the delivery tube will be air.
- When the soap bubbles start to float up, hold a burning candle to them and listen carefully for the sound they make when they pop. Do not hold the candle to the end of the delivery tube.
- When the magnesium stops reacting and no further hydrogen bubbles are released, extinguish the candle and set it aside.
- Disassemble the experiment and test the pH of the reaction mixture. Record the pH value.
RESULTS AND OBSERVATIONS:
Record your results in the following table:
|
pH of the 1M hydrochloric acid before the reaction |
|
|
pH of the mixture after the reaction |
Learners should note that there is a 'pop' sound when the candle bursts the bubbles. They should note that when HCl is added to the magnesium pieces, the solution bubbles as the gas is produced.
CONCLUSIONS:
What conclusions can be made from the results of your investigation? Rewrite your hypothesis, but change it to reflect your findings if they are different from what you predicted earlier.
QUESTIONS:
Bubbles formed on the surface of the magnesium pieces.
Bubbles came out of the end of the gas delivery tube.
The gas in the soap bubbles is less dense than air. NOTE: Learners may say 'lighter' than air; you can use the opportunity to remind them that less dense substances will float on substances of higher density.
Hydrogen gas (H2). Hydrogen is less dense than air so it made soap bubbles and floated upwards which made the characteristic 'pop' sound when a candle was brought near to the bubbles.
The pH increased.
When the pH increases, it means there is less acid in the solution. The hydrochloric acid was being used up in the reaction with magnesium.
Learner dependent answer.
In our investigation hydrochloric acid reacted with magnesium (a metal). Our next task is to write an equation for the reaction. We will begin by writing a general equation and end with one that matches the reaction that we have just investigated.
General equation for the reaction of an acid with a metal
The general word equation for the reaction between an acid and a metal is:
acid + metal → salt + hydrogen gas
The type of salt that forms will depend on the specific metal and acid which are used in the reaction.
A video and simulation that teaches you more about the reactions of acids with metals http://www.bbc.co.uk/bitesize/ks3/science/chemical_material_behaviour/acids_bases_metals/activity/
Equations for the reaction between magnesium and hydrochloric acid
Now, let's write equations for our actual reaction from the last investigation.
Writing the chemical equation
The following steps will guide you:
HCl
Magnesium (Mg)
H2
Write what remains of the acid (HCl) after we have taken away the H to make H2. (Remember we need two H to make one H2).
2 Cl
MgCl2
-
Now, let's write the reaction; first the reactants, then the products:
2 HCl + Mg → MgCl2+ H2
Let's check quickly if the reaction is balanced.
How many H on the left and right? Are they balanced?
2 H left and 2 H right. The H's are balanced.
How many Cl on the left and right? Are they balanced?
2 Cl left and 2 Cl right. The Cl's are balanced.
How many Mg on the left and right? Are they balanced?
1 Mg left and 1 Mg right. The Mg's are balanced.
Since the numbers of each type of atom is the same on either side of the equation, we can confirm that it is balanced.
Finally, let's use the chemical equation to write a word equation for the reaction:
hydrochloric acid + magnesium → magnesium chloride + hydrogen gas
Coke cans made from aluminium (a metal) react with an acid and a base (video)
Chemist or Pharmacist?
This section is not for assessment purposes and you may be inclined to leave it out. However, we strongly encourage you to give your learners the opportunity to discover the applications of what they are learning in class in the world around them, even if only as a homework exercise. It is very important for learners to realise that what they learn in class extends far beyond the walls of your classroom. Encourage them to be curious!
When people hear that someone is a 'chemist', they often confuse this with being a 'pharmacist'. In some countries the terms 'chemist' and 'pharmacist' are even used to describe the same kind of person. In South Africa the two words have different meanings. But what is the difference between being a chemist and being a pharmacist?
Look up these careers to identify the main difference between a chemist and a pharmacist and summarise them below:
-
Chemists:
Chemists are people who have studied chemistry and can use their specialist knowledge of chemical reactions to produce new materials and compounds. These could be new medicines, innovative building materials, new fuels that do not harm the environment and many others.

-
Pharmacists:
Pharmacists also study chemistry, but combine this with other subjects like pharmacology, physiology and biochemistry. Pharmacists are health professionals and have specialist training in the health sciences as well as the chemical sciences. Their key responsibility is to ensure the safe and effective use of pharmaceutical drugs. They use their knowledge of medicines and the human body to dispense prescriptions from a licensed pharmacy. Job opportunities for pharmacists also include clinical services, reviewing medications for safety and efficacy and providing drug information where it is needed.

Other careers in chemistry
This is an optional activity, which is not for assessment. A suggestion is that if you do not have time to do it in class, learners should still be encouraged to do it outside of class as it is important that they see how and where chemistry can take them after school.
A useful site to find out more about some chemistry-related careers http://www.acs.org/content/acs/en/careers/whatchemistsdo/careers.html
INSTRUCTIONS:
- Below is a list of different careers that all use chemistry in some way. Have a look through the list and then select the five careers you find most interesting.
- Do an internet search to find out what each career is.
- Write a one line description of this career.
- If there is a career that really interests you, draw a smiley face next to it and be sure to do some extra reading around the topic and where chemistry might take you! Find out what level of chemistry you will need for this particular career.
- There are many other careers besides the ones listed here which use chemistry in some way, so if you know of something else which is not listed here and it interests you, follow your curiosity and discover the possibilities!
Some careers involving chemistry:
- Agricultural chemistry
- Biochemistry
- Biotechnology
- Chemical education/teaching
- Dentistry
- Environmental chemistry
- Forensic science
- Food science/technology
- Geneticist
- Geochemistry
- Materials science
- Medicine and medicinal chemistry
- Mining
- Oil and petroleum industry
- Organic chemistry
- Oceanography
- Patent law
- Pharmaceuticals
- Space exploration
- Zoology
Your descriptions of the careers you are interested in:
Great scientists (including chemists) are observant, curious, patient and eager to learn more about their field every day.
In this chapter we have studied the reaction of hydrochloric acid with magnesium, as an example of a reaction between an acid and a metal.
10 reasons to love science! (video)
Summary
- An acid will react with a metal to form a salt and hydrogen gas.
- The general word equation for the reaction between an acid and a metal is as follows:
-
acid + metal → salt + hydrogen
-
Concept map
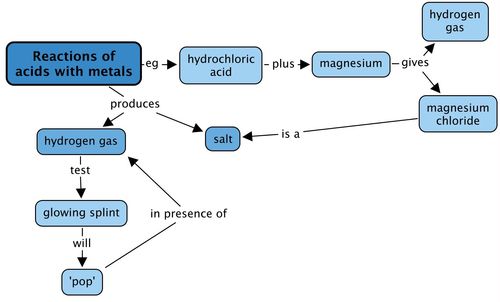
This was quite a short chapter, so the concept map has been left blank for you to do your own. Be sure to include something about the test for hydrogen.

This is an example of what learners might produce:
Revision Questions
Fill in the missing words in these sentences. Write the word on the line below. [3 marks]
-
When an acid reacts with a metal, a salt and _____ gas forms.
-
A molecule that consists of two atoms bonded together is called a _____ molecule.
-
The scientific quantity represented by the mass of a substance in a given volume is called the _____ of that substance.
- hydrogen
- diatomic
- density
Learner's paragraph should contain at least the following:
- Hydrogen gas is less dense than air.
- Substances of lesser density always float on substances of greater density.
Learner's paragraph should contain at least the following:
- The first sign to look out for is bubbles. The presence of bubbles signals that a gas is formed during the reaction.
- To confirm whether the gas is hydrogen, collect a small amount in a test tube. Hold a glowing splint at the opening of the test tube when you release the gas. If the gas ignites with a characteristic 'pop' sound, we will know it is hydrogen.
The pH will increase. In the reaction, the acid is changed into something else that is not an acid. That means the pH must increase.
Complete the following table by providing the missing equations for the reaction between hydrochloric acid and magnesium [6 marks]
|
Word equation |
|
|
Chemical equation |
|
|
General equation |
|
Word equation |
hydrochloric acid + magnesium → magnesium chloride + hydrogen gas |
|
Chemical equation |
2 HCl + Mg → MgCl2 + H2 |
|
General equation |
acid + metal → salt + hydrogen |
Complete the following table by providing the missing equations for the reaction between hydrochloric acid and zinc [4 marks]
|
Word equation |
|
|
Chemical equation |
2 HCl + Zn → ZnCl2 + H2 |
|
General equation |
|
Word equation |
hydrochloric acid + zinc → zinc chloride + hydrogen gas |
|
Chemical equation |
2 HCl + Zn → ZnCl2 + H2 |
|
General equation |
acid + metal → salt + hydrogen |
We have looked at many different chemical reactions this term. As a summary, complete the following table by giving the general equations in words for each of the chemical reactions in the second column, and provide an example for each type as a balanced chemical equation in the third column. [18 marks]
|
Type of chemical reaction |
General word equation |
Example (balanced equation) |
|
metals with oxygen |
||
|
non-metals with oxygen |
||
|
acids with metal oxides |
||
|
acids with metal hydroxides |
||
|
acids with metal carbonates |
||
|
acids with metals |
Only one example has been provided in this table as an example of what learners might write. There are however other suitable reactions which they have also learnt about this term. You must check that the reactions they provide are balanced.
The mark allocation is 1 mark for each of the general word equations and 2 marks for the example (only 1 mark if it is not correctly balanced).
|
Type of chemical reaction |
General word equation |
Example (balanced equation) |
|
metals with oxygen |
metal + oxygen → metal oxide |
2Mg + O2→ 2MgO |
|
non-metals with oxygen |
non-metal + oxygen → non-metal oxide |
C + O2→ CO2 |
|
acids with metal oxides |
acid + metal oxide → salt + water |
2HCl + MgO → MgCl2 + H2O |
|
acids with metal hydroxides |
acid + metal hydroxide → salt + water |
HCl + NaOH → NaCl + H2O |
|
acids with metal carbonates |
acid + metal carbonate → salt + carbon dioxide + water |
2HCl + CaCO3→ CaCl2 + CO2 + H2O |
|
acids with metals |
acid + metal → salt + hydrogen gas |
2HCl + Mg → MgCl2 + H2 |
Total [38 marks]
