A possible answer is: What will happen to the pH of the solution if we add vinegar to baking soda?
Reactions of acids with bases
Chapter overview
2 weeks
The central challenge of this chapter is to establish that acid-base reactions are exchange reactions. A fragment of the acid is exchanged with a fragment of the base and a salt and water are the resulting products of the reaction. The type of salt that forms depends on the identities of the acid and the base that were combined during the reaction.
Once learners understand this, they have taken an important step to understanding acid-base chemistry. We will spend some time developing a frame for explaining this at the start of the chapter, to which we will return frequently.
In light of the fact that learners have yet to learn about cations and anions, we have considered it pedagogically justifiable to make the following simplifications to currently accepted acid-base theory, in order to bring the concept of exchange across to the learners:
-
Acids can be thought of as contributing H (instead of H+); and
-
Bases can be thought of as contributing O or OH (instead of O2- and OH-).
-
Water (H2O) is a combination of 2 H and 1 O, or alternatively 1 H and 1 OH.
We are well aware that writing H + OH → H2O has no meaning in science and for this reason we have avoided this usage in the text. But we do consider the use of simplified symbols (H instead of H+ and so forth) to have an advantage over their scientifically correct (but potentially confusing) counterparts in this context.
There is also a danger that misconceptions and sloppy usage of symbols may result further down the line, when simplifying in this way. However, we feel these risks are counterbalanced by the greater likelihood of learners understanding the concept of exchange if the symbols they work with are not cluttered with additional information - like the charges on the ions - that have no meaning for them yet.
Other skills that will be reinforced in this chapter are:
- writing chemical formulae;
- converting between word equations and chemical equations; and
- balancing chemical equations.
A word of caution: Acid-base reactions are neutralisation reactions. However, this does not mean that the mixture of an acid with a base will be a neutral solution and you should avoid language that reinforces this notion. Even if equivalent quantities (stoichiometric quantities) of the acid and base are mixed - which would imply that both have been neutralised - the resulting solution will only be neutral (i.e. pH = 7) under very special circumstances. The reason is that not all salts are 'neutral substances'; in fact most salts have acid-base properties of their own. The chemistry required for learners to understand this is beyond them at this stage and will only be dealt with in Physical Sciences in Grade 12. Our suggestion is that you simply refrain from calling salts 'neutral substances'. If questions arise around the issue you could point out that the salts they will encounter in this chapter may be neutral substances, but that this is not true of all salts.
Take note that although there is no section specifically named 'Applications' as indicated in CAPS, this content has rather been dealt with under other sections where it is more appropriate.
6.1 Neutralisation and pH (1.5 hours)
|
Tasks |
Skills |
Recommendation |
|
Investigation: The reaction between vinegar and baking soda |
Hypothesising, measuring, preparing, observing, comparing, recording, plotting graphs |
CAPS suggested |
|
Activity: CO2 bubbled through water |
Observing, measuring, comparing |
Optional |
|
Activity: What is acid rain? |
Observing, reading, researching, interpreting, analysing, summarising |
CAPS suggested |
6.2 The general reaction of an acid with a metal oxide (1.5 hours)
|
Tasks |
Skills |
Recommendation |
|
Investigation: The reaction between magnesium oxide and hydrochloric acid |
Hypothesising, preparing, observing, measuring, recording, plotting graphs |
CAPS suggested |
|
Activity: Writing the chemical equation |
Writing and balancing chemical equations |
Optional (Suggested) |
6.3 The general reaction of an acid with a metal hydroxide (1.5 hours)
|
Tasks |
Skills |
Recommendation |
|
Investigation: The reaction between sodium hydroxide and hydrochloric acid |
Hypothesising, preparing, measuring, observing, measuring, recording, plotting graphs |
CAPS suggested |
|
Activity: Writing the chemical equation |
Writing and balancing chemical equations |
Optional (Suggested) |
6.4 The general reaction of an acid with a metal carbonate (1.5 hours)
|
Tasks |
Skills |
Recommendation |
|
Investigation: The reaction between calcium carbonate (chalk) and hydrochloric acid |
Hypothesising, preparing, comparing, measuring, recording, plotting graphs |
CAPS suggested |
|
Activity: Writing the chemical equation |
Writing and balancing chemical equations |
Optional (Suggested) |
- What is the reaction between an acid and a base called?
- What happens to the pH when an acid and a base are mixed?
- Does the reaction between an acid and a base always give a neutral mixture, in other words a mixture with pH = 7?
- Which factors will determine the pH of the final solution when an acid and a base are mixed?
- Is there a way to predict which classes of compounds will tend to be acids and which will tend to be bases?
- Are metal oxides, metal hydroxides and metal carbonates acidic or basic? Which pH range will their solutions fall into?
- What products can we expect when a metal oxide, a metal hydroxide or a metal carbonate react with an acid?
- Are there general equations to explain these reactions?
- How does acid rain form?
Neutralisation and pH
- neutralisation reaction
- neutralise
- neutral solution
- potency
- detour
- exchange reaction
- laboratory acids
- corrosive
- acid rain
In the previous chapter we learnt about a new concept, namely pH. If we want to know whether something is an acid or a base, we can measure its pH:
- Acids have pH values below 7. The lower the pH value, the more strongly acidic the substance.
- Bases have pH values above 7. The higher the pH value, the more strongly basic the substance.
- Neutral substances have pH equal to 7.
Another useful thing we learnt in the previous chapter is that we can use universal indicator to measure the pH of a solution. Universal indicator has different colours at different pH values. Below is a colour chart showing the range of colours for universal indicator and the pH values they correspond to. You will need it for all the activities of this chapter, because we are going to do lots of pH measurements!

Can you remember how we used the universal indicator paper in the previous chapter? Here are some suggestions for the investigations of this chapter:
- Before you start, place 1-cm lengths of universal indicator paper on a sheet of white paper, like this:
Later, if you want to write down a note or an observation, you can do so directly on the paper and copy it to your workbook afterwards.
-
Instead of dipping the paper in the solutions you are testing, use a glass rod or drinking straw to transfer a drop of the test solution to the indicator paper.
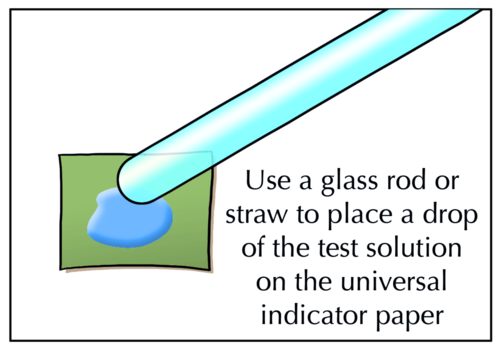
For some of the investigations in this chapter, you will be using droppers or syringes to measure out quantities. Tell learners that they may not use droppers or syringes to squirt water at other learners! There are many reasons why this is not a good idea. The most important reason is that the dropper or syringe may contain acid, that could end up in someone's eye where it could cause permanent damage or even blindness. So, squirting each other with the droppers or syringes is not allowed.
What is neutralisation?
What do you think would happen if we mixed an acid and a base?
Get learners to discuss this in class or in small groups. Allow them to speculate and guide them to recall their Grade 7 learning: An acid will lose its potency when it is mixed with a base and vice versa. So the acid will be weakened by the base and the base will be weakened by the acid. 'Weaken', however, is a term best avoided, because 'weak' and 'strong' have very specific meanings when speaking about acids and bases. In a sense their acid-base properties will be destroyed, because they will be converted to products that won't be acids or bases. (Often the salt that results from the reaction between an acid and a base will have acid-base properties of its own, but we will not be discussing that now.)
We are going to do an investigation to find out. We are going to mix vinegar with baking soda. But first, a little revision: is vinegar an acid or a base? If you are not sure, imagine putting a drop of vinegar on your tongue. What would it taste like?
It would taste sour, therefore it is an acid.
Is baking soda an acid or a base? If you are not sure, turn back to the previous chapter and look at the activity 'The pH scale'.
Baking soda is a base.
The reaction between vinegar and baking soda
Quantities for this investigation are as follows: Every 1 g of baking soda will require approximately 15 ml of vinegar for complete neutralisation. We recommend that you measure out 1 teaspoon of baking soda and approximately 50 ml vinegar for each group.
The purpose of this experiment is to investigate how the pH changes when vinegar is added to baking soda.
INVESTIGATIVE QUESTION(S):
OVERVIEW OF THE INVESTIGATION:
In the range pH > 7
The pH will decrease.
HYPOTHESIS:
When we add vinegar to baking soda, the pH of the mixture will decrease.
MATERIALS:
- baking soda
- vinegar
- water
- glass beaker or small yoghurt tub
- universal indicator paper (cut into 1 cm strips)
- sheet of white printer paper
- plastic teaspoon
METHOD:
- Prepare the universal indicator paper by neatly placing five 1-cm pieces underneath each other on the sheet of paper.
- Place one teaspoon of baking soda in the beaker or yoghurt tub.
- Add approximately 10 teaspoons of water to the baking soda.
- Use the teaspoon to stir the solution until all the baking soda has dissolved. We will be calling this the 'test solution' from now on.
- Transfer one drop of the test solution to the first piece of universal indicator paper using the teaspoon or a straw.
- Compare the colour of the paper with the colour guide given at the start of the chapter, to find the pH of the solution. Record this pH in your results table.
- Add 1 teaspoon of the vinegar to the test solution. Stir it gently and transfer another drop of the solution to a fresh strip of the universal indicator.
- Read the pH of the solution off the colour guide and record it in your results table.
- Repeat steps 6 and 7 until the pH of the test solution drops below 7. You may need more than 5 pieces of universal indicator paper.
RESULTS:
Present your results in a neat table. Use appropriate headings for your table. 'Number of teaspoons of vinegar added' and 'Colour of the universal indicator paper' and 'pH of the test solution' are suggested headings for your columns.
Draw a line graph to illustrate your results. What will be on the x-axis and what will be on the y-axis? Give your graph a heading.
Learners must draw a graph with the 'number of teaspoons of vinegar added' on the x-axis (independent variable) and the pH of the solution on the y-axis (dependent variable).
CONCLUSIONS:
What conclusions can be made from the results of your investigation? Here you can rewrite your hypothesis, but change it to reflect your findings if they are different from what you predicted earlier.
Were you able to confirm or reject your hypothesis?
In this investigation, you probably noticed that the pH of the mixture dropped every time you added more vinegar to the baking soda! Why did this happen?
When an acid and a base are mixed (in the right amounts), they will neutralise each other. That means that, together, they will change into something that is neither an acid nor a base. So, the acid will lose its 'acidity' and the base will lose its 'basicity'.
What have we learnt so far? We have learnt that acids and bases neutralise each other:
-
If we add a base to an acid, the pH of the resulting solution will increase, because the acid will lose some of its potency.
- If we add an acid to a base, the opposite will happen. The pH will decrease, because the base will lose some of its potency.
What are the products of an acid-base reaction? Can we predict what they will be?
The products of acid-base reactions
In order to understand how an acid-base reaction works, we have to take a quick detour and say something about exchange reactions. Acid-base reactions are exchange reactions.
In the reaction below, two substances AB and CD are undergoing an exchange reaction:
AB + CD → AD + CB
Can you see that A and C have exchanged partners so that A is now combined with D, while C combined with B?
What does this have to do with acids and bases? Well, acids and bases undergo exchange reactions too. Here are some examples. See if you can figure out which parts have exchanged with which.
Example 1
HCl + NaOH → NaCl + HOH
In the above equation HOH should actually be written: H2O. The reaction equation becomes:
HCl + NaOH → NaCl + H2O
or, in words:
hydrochloric acid + sodium hydroxide →sodium chloride + water
In this example, the following happened:
- the acid gave its H towards making a water molecule;
- the base gave OH towards making a water molecule; and
- the Na from the base and the Cl from the acid combined to form a salt.
Example 2
2 HCl + MgO → MgCl2 + HOH
In the above equation HOH should actually be written: H2O. The reaction equation becomes:
2 HCl + MgO → MgCl2 + H 2O
or, in words:
hydrochloric acid + magnesium oxide →magnesium chloride + water
In this example, the following happened:
- the acid gave 2 H towards making a water molecule;
- the base gave OH towards making a water molecule; and
- the Mg from the base and the 2 Cl from the acid combined to form a salt.
Acid-base reactions always produce water and a salt. In both of the examples above the general equation was:
acid + base → salt + water
There is one class of acid-base reactions that produces an additional product, but we will learn more about that later.
In Grade 11 you will learn that the mechanisms of these reactions are actually slightly more complex than this, but for now, understanding it at this level is good enough.
Which laboratory acids should we know about?
When we investigated acids and bases in the previous chapter, we considered only household acids like lemon juice and vinegar. There are a few laboratory acids that you should know the names and formulae of and they have been listed in the following table:
|
Name of the acid |
Formula of the acid |
|
hydrochloric acid |
HCl |
|
nitric acid |
HNO3 |
|
sulfuric acid |
H2SO4 |

These acids are very corrosive, even when they have been diluted with water and should always be handled with great care.
What happens if you put a burger in concentrated hydrochloric acid? (video)
In the next sections will discuss the classes of substances that are typically acids or bases. Two important things to remember are the following:
- Non-metal oxides form acidic solutions when they are dissolved in water.
- Metal oxides, metal hydroxides and metal carbonates all form basic solutions when they are dissolved in water.
First, we will look at the non-metal oxides.
Non-metal oxides form acidic solutions
Can you name a few non-metal oxides? Write down their formulae. If you are not sure you can take a peek at the Periodic Table and pick a few non-metals from the right-hand side of the table. Add oxygen and you have a non-metal oxide!
CO2 and SO2
How do we know that non-metal oxides form acidic solutions? Experiments have shown this.
You may not know this, but when CO2 gas is bubbled through water some of it dissolves in the water to form carbonic acid. Here is the reaction equation:
CO2 + H2O → H2CO3
To see this happen, try the following quick activity.
CO2 bubbled through water
A variation of this activity is if you have liquid universal indicator, you can add it to the tap water at the start to show the pH is 7 (it should be green). Then as you blow into the water, the universal indicator will change colour. Make sure you use a clear glass so learners can observe the colour change as it becomes more yellow. This links to the next activity on acid rain and how it forms.
MATERIALS:
- tap water
- glass
- straw
- indicator paper
INSTRUCTIONS:
We could measure the pH of the water with universal indicator paper.
-
Now exhale into the water using a straw. Your breath contains CO2 and some of this will dissolve in the water if you carry on doing this for a few minutes.

The pH will be below 7.
Carbonic acid is added to soft drinks to make it fizzy. The carbonic acid decomposes and forms carbon dioxide (CO2)
The pH of the solution is now below 7 because it contains carbonic acid (H2CO3). Carbonic acid is not a very strong acid, but still acidic enough to have a pH lower than 7.
When sulfur dioxide (a gas) is bubbled through water it dissolves in the water to form an acid called sulfurous acid:
SO2 + H2O → H2SO3
These are two of the reactions that produce a phenomenon called acid rain. SO2 and CO2 are released as waste products from factories and power stations. For example, burning wood and fossil fuels releases carbon dioxide and sulfur dioxide into the atmosphere. These gases then dissolve in water droplets in the atmosphere to form acids, in a similar way that the CO2 in your breath dissolved in the water in the last activity to produce an acidic solution. When it rains, these acids are present in the raindrops that fall back to earth. Sulfurous acid (H2SO3) is strong enough to damage plant life and to acidify water sources.


For the next activity, you have to do some research on acid rain.
Volcanoes also release non-metal oxides into the air (mainly SO2) that can contribute to acid rain.
What is acid rain?
INSTRUCTIONS:
- Study the diagram showing how acid rain forms.
- Do some extra reading and research about acid rain.
- Answer the questions about acid rain.
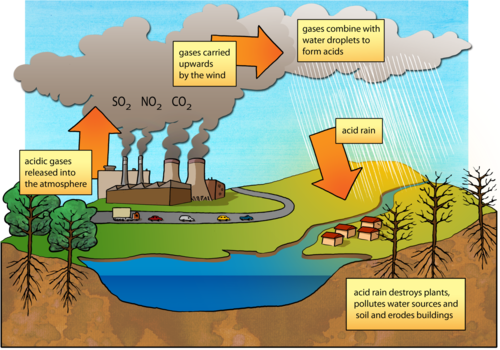
QUESTIONS:
They are sulfur dioxide (SO2), carbon dioxide (CO2) and nitrogen dioxide (NO2).
The main sources of these gases which contribute to acid rain are from human activity, such as electricity generation in fossil fuel power plants (especially coal), factories emitting smoke and the exhaust fumes from motor vehicles. Acid rain can also occur due to natural phenomena, such as volcanoes which emit sulfur dioxide into the atmosphere. Some processes in the ocean and in wetlands also produce the gases which form acids.
SO2 + H2O → H2SO3
CO2 + H2O → H2CO3
Sulphurous acid and carbonic acid.
The impacts include:
- damage of plant life, both wilderness areas and also crops, depending on where the rain falls
- the rain leaches into soil and makes it more acidic; this kills microorganisms living in the soil, damages plants further by contaminating soil water
- the rain can fall into various water sources and also run off into water sources such as rivers, lakes and dams; this causes the water to become more acidic; aquatic animals and plants can die; human water sources become too acidic as well
Acids are corrosive and so they can corrode surfaces over time.
Learners need to justify their answers. They may say that it helps the local environment as the gases are carried further away and therefore do not pollute the town or city that the factory is in or near. But this does not do anything to minimize the acid rain that could potentially form as the same amount of gases are still emitted; they are just carried further away. The acid rain therefore can still form and fall on the vegetation and areas outside of the towns and cities.
There are several solutions to minimizing the formation of acid rain. For example, coal-powered stations can use filters and other processes in their smoke towers to remove sulfur gases before the smoke is released into the atmosphere. Countries can take bigger steps by signing treaties to reduce their sulfur and other greenhouse gas emissions. The move towards using renewable energy sources will also help to reduce the reliance on coal and other fossil fuels, thereby reducing the emission of acid-producing gases into the atmosphere.
We have now learnt about non-metal oxides, but what about metal oxides? What kind of solutions do they form in water? We will find out more about them and other metal compounds in the next section.
Metal oxides, metal hydroxides and metal carbonates form basic solutions
Metal oxides
Do you remember learning about some of the metal oxides in Chapter 3? We already learnt these rules to write the formulae of metal oxides.
-
Metal oxides from group 1 on the Periodic Table will have the formula M2O, where M represents any metal.
Can you write two examples? Look at the Periodic Table at the front of the book, pick any two metals from group 1 and write their formulae using this rule.
Any two of the following: Li2O, Na2O, K2O, Rb2O, Cs2O
-
Metal oxides from group 2 will have the formula MO.
Can you write 2 examples?
Any two of the following: BeO, MgO, CaO, SeO, BaO
What do you think the pH will be of a solution of a metal oxide in water?
The pH will be above 7.
The next class of compounds that form basic solutions in water are the metal hydroxides.
Metal hydroxides
A metal hydroxide forms when a metal reacts with water. A metal hydroxide has the general formula MOH or M(OH)2. In the formula, M represents a metal atom, O represents an oxygen atom and H represents a hydrogen atom.
To know whether the MOH or M(OH)2 will be the correct formula, here are two simple rules for you to remember:
-
Metal hydroxides from group 1 on the Periodic Table will have the formula MOH.
Can you write two examples? Look at the Periodic Table at the front of the book, pick any two metals from group 1 and write their formulae using this rule.
Any two of the following: LiOH, NaOH, KOH, RbOH, CsOH.
-
Metal hydroxides from group 2 will have the formula M(OH) 2.
Can you write two examples?
Any two of the following: Be(OH)2, Mg(OH)2, Ca(OH)2, Sr(OH)2, Ba(OH)2.
What do you think the pH will be of a solution of a metal hydroxide in water?
The pH will be above 7.
The final class of compounds that forms basic solutions in water is the metal carbonates. Baking soda is a special kind of carbonate, called a bicarbonate (or hydrogen carbonate). You may remember that it was one of the bases we tested with universal indicator earlier.
Metal carbonates
A metal carbonate has the general formula MCO3 or M2CO3. In the formula, M represents a metal atom, C represents a carbon atom and O represents an oxygen atom.
To know whether the MCO3 or M2CO3 will be the correct formula, there are two simple rules to remember:
-
Metal carbonates from group 1 on the Periodic Table will have the formula M2CO3.
Can you write two examples?
Any two of the following: Li2CO3, Na2CO3, K2CO3,Rb2CO3, Cs2CO3.
-
Metal hydroxides from group 2 will have the formula MCO3.
Can you write two examples?
Any two of the following: BeCO3, MgCO3, CaCO3, SrCO3, BaCO3.
What do you think the pH will be of a solution of a metal carbonate in water?
The pH will be above 7
In the next sections we will be investigating real reactions!
The general reaction of an acid with a metal oxide
- metal oxide
In the previous section we learnt about two classes of oxides, namely metal oxides and non-metal oxides. Here is what we know about them so far:
- Metal oxides are formed from the reaction between a metal and oxygen. Metal oxides are basic. When we dissolve them in water, they form solutions with pH values above 7.
- Non-metal oxides are formed from the reaction between a non-metal and oxygen. Non-metal oxides are acidic. When they dissolve in water, they form solutions with pH values below 7.
Here is the same summary, in table form, with some examples added:
|
Metal oxides |
Non-metal oxides |
|
metal + oxygen → metal oxide |
non-metal + oxygen → non-metal oxide |
|
basic |
acidic |
|
pH > 7 |
pH |
|
Examples: Li2O, Na2O, MgO, CaO |
Examples: CO2, SO2, NO2, P2O5 |
In this section, we are going to learn about the reactions between metal oxides and acids.
The reaction between magnesium oxide and hydrochloric acid
This investigation requires magnesium oxide from the reaction when magnesium ribbon burns in oxygen. If you have set some aside from the earlier activity 'The reaction of magnesium with oxygen' (Chapter 3), learners can use it for this investigation. If you did not, you can easily repeat that demonstration to produce more white magnesium oxide powder for this next investigation. This investigation is also suitable to scale up as a demonstration.
The purpose of this investigation is to:
- test whether a solution of magnesium oxide in water is acidic, basic or neutral; and
-
determine whether the reaction between an aqueous solution of magnesium oxide and hydrochloric acid is a neutralisation reaction.
INVESTIGATIVE QUESTION(S):
What are the questions you hope to answer with this investigation? Write them in the space below. There are a few words to start you off.
When magnesium oxide is dissolved in water, will the resulting solution be acidic, basic or neutral?
When a solution of magnesium oxide is treated with hydrochloric acid, will the pH of the mixture increase, decrease, or stay the same?
OVERVIEW OF THE INVESTIGATION:
In the range pH > 7
The pH will decrease
HYPOTHESIS:
What are your predictions? Your hypothesis should be a prediction of the finding(s) of the investigation. You should write it in the form of a possible answer to the investigative question(s). Here are a few words to start you off:
When magnesium oxide is dissolved in water, the resulting solution will be basic (have a pH > 7).
When a solution of magnesium oxide is treated with hydrochloric acid, the pH of the mixture will decrease.
MATERIALS:
- magnesium oxide powder
- water
- universal indicator paper (cut into 1 cm strips)
- white tile or sheet of white printer paper
- glass rod (or plastic straw)
- test tube
- dropper
- hydrochloric acid (HCl) solution (0.1 M)
Notes for the investigation:
-
Learners must not dilute hydrochloric acid themselves as it reacts strongly with water. Make sure to add the acid slowly to the water and NOT the other way around.
- To prepare 0.1 M HCl solution, carefully add approximately 10 ml concentrated hydrochloric acid (33% or 11 M) to 1 liter of tap water. It is recommended that you wear safety goggles and protective gloves during this step and that you rinse away any acid spills with cold tap water. Since this is just a qualitative experiment, it is not necessary to use distilled water for the solution. It is also not required that you measure the volumes with extreme accuracy.
- The following guide will help you to determine quantities: The magnesium oxide prepared from a 1 cm length of magnesium ribbon will require approximately 8 ml of 0.1 M hydrochloric acid for complete neutralisation. If the learners work in small groups and each group dissolves a small quantity of MgO (the size of a match head) in 2 ml of water, they will only need a few drops of HCl solution to neutralise all the MgO.
-
If you have universal indicator solution, this will work very nicely as you can observe the colour changes as you add the drops.
-
If you decide to give the learners droppers to measure out the HCl, you will have to enforce very strict rules for handling the droppers. Learners find the temptation to squirt water at each other very difficult to resist and they must be made aware of the hazards of accidentally squirting acid at another learner.
- Remind learners to use the colour guide for universal indicator provided at the start of the chapter. If you have the budget, a good idea would be to make a number of colour photocopies of the chart and to have them laminated so they will last longer.
- Remind learners to prepare a table for their results beforehand.
METHOD:
- Prepare the universal indicator paper by neatly placing five 1 cm pieces in a column on the white tile or sheet of printer paper.
- Place a small quantity (the size of a match head) of the magnesium oxide in a test tube.
- Add approximately 2 ml of tap water to dissolve most of the magnesium oxide.
-
Use the glass rod (or plastic straw) to stir the solution until most the magnesium oxide has dissolved. We will be calling this the test solution from now on.
- Transfer one drop of the test solution to the first piece of universal indicator paper.
- Compare the colour of the paper with the colour guide to find the pH of the solution.
- Record this pH in the table you prepared beforehand.
- Add 10 drops of the hydrochloric acid solution to the test solution. Stir it gently and transfer another drop of the solution to a fresh strip of the universal indicator.
- Read the pH of the solution off the colour guide and record it in your table.
- Repeat steps 3 and 4 until the pH of the test solution drops below 7. You may need more than 5 pieces of universal indicator paper.
RESULTS:
Learner-dependent answer
-
Draw a graph of your results. Here are some hints to help you decide which variable to put on which axis:
What is your independent variable? (Which variable did you change?) This goes on the x-axis.
Number of drops of HCl
What is your dependent variable? (Which variable did you measure?) This goes on the y-axis.
pH
The number of drops of HCl should be on the x-axis of the graph and pH should be on the y-axis. There should be a general trend downwards (since acid is added to a base, we can expect the pH to drop), but it should not be linear. This experiment is a very rudimentary 'titration' and an example of a titration curve from this experiment is given here:
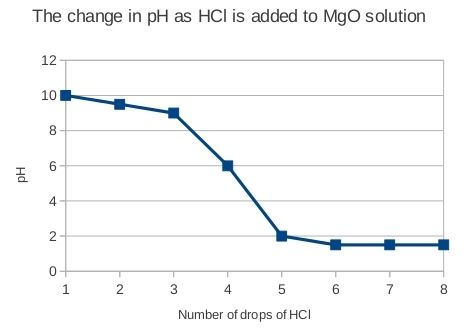
It is therefore not expected that learners' curves will be linear, but rather that there will be a gradual decline in pH at first, followed by a rapid drop when all the base has been neutralised. After this the curve levels out again.
CONCLUSIONS:
What conclusions can be made from the results of your investigation? Rewrite your hypothesis, but change it to reflect your findings if they are different from what you predicted earlier.
Were you able to confirm or reject your hypotheses?
Now that we have investigated a reaction between a metal oxide (MgO) and an acid (HCl), we can write an equation for the reaction. We will begin by writing a general equation and end with one that matches the reaction that we have just investigated.
A video showing the reaction of copper(II)oxide with hydrochloric acid
General equation for the reaction of an acid with a metal oxide
Can you remember learning that an acid-base reaction is an exchange reaction? We learnt that:
- The acid contributes H towards making a water molecule;
- The base contributes O or OH towards making a water molecule; and
-
Whatever is left of the the acid and the base after making a H2O molecule, combines to form a salt.
The general word equation for the reaction between an acid and a base is:
acid + base → salt + water
Since the base in our reaction is a metal oxide we can write:
acid + metal oxide → salt + water
This is the general word equation for the reaction between an acid and a metal oxide. The type of salt that forms will depend on the specific acid and metal oxide which were used in the reaction.
Equations for the reaction between magnesium oxide and hydrochloric acid
Now we are going to learn how to write equations for our actual reaction.
Writing the chemical equation
The following steps will guide you:
HCl
Magnesium oxide (MgO)
Now,let's try to predict the products of the reaction. We know that water will be one of the products.
Mg
Write what remains of the acid (HCl) after we have taken away the H (to make water). (Remember we need two H to make one H2O).
2 Cl (we used 2 HCl)
MgCl2
Now, let's put it all together, first the reactants, then the products:
2 HCl + MgO → MgCl2 + H2O
-
Let's check quickly if the reaction is balanced.
How many H atoms on the left hand side and on the right hand side? Are they balanced?
2 H atoms left and 2 H atoms right. The H's are balanced.
How many Cl atoms on the left hand side and on the right hand side? Are they balanced?
2 Cl atoms left and 2 Cl atoms right. The Cl's are balanced.
How many O atoms on the left hand side and on the right hand side? Are they balanced?
1 O atoms left and 1 O atoms right. The O's are balanced.
Since the numbers of each type of atom is the same on either side of the equation, we can confirm that it is balanced.
Finally, let's use the chemical equation to write a word equation for the reaction:
hydrochloric acid + magnesium oxide → magnesium chloride + water
In the next section we are going to look at the reactions between acids and metal hydroxides.
The general reaction of an acid with a metal hydroxide
- metal hydroxide
We will start this section with an investigation to illustrate the reaction between an acid and a metal hydroxide.
The reaction between sodium hydroxide and hydrochloric acid
Notes for the investigation:
- The same cautions regarding droppers and syringes apply to this activity. You will need to enforce very strict rules for handling these items or learners may find the temptation to squirt water at each other very difficult to resist.
- Remember to provide learners with a colour guide for universal indicator, if you have this.
- Remind learners to draw a results table before they start the experiment.
The purpose of this investigation is to:
- test whether sodium hydroxide is acidic or basic; and
- determine whether the reaction between sodium hydroxide and hydrochloric acid is a neutralisation reaction.
INVESTIGATIVE QUESTION(S):
Here are some ideas:
- When sodium hydroxide is dissolved in water, will the resulting solution be acidic, basic or neutral?
- When a solution of sodium hydroxide is treated with hydrochloric acid, will the pH of the mixture increase, decrease or stay the same?
- Will it be possible to neutralise all the sodium hydroxide by adding hydrochloric acid?
OVERVIEW OF THE INVESTIGATION :
In the range pH > 7
The pH will decrease
HYPOTHESIS:
Some ideas:
- Sodium hydroxide solution will have a pH greater than 7.
- When a solution of sodium hydroxide is treated with hydrochloric acid, the pH of the mixture will decrease.
- By adding hydrochloric acid to the sodium hydroxide solution, it should be able to decrease the pH to 7 and even below 7.
MATERIALS:
- sodium hydroxide solution (0.1 M)
Prepare 0.1 M NaOH solution by dissolving approximately 4 g of NaOH pellets in 1 liter of cold tap water. Wear safety goggles and gloves since there is a chance the sodium hydroxide solution could splash up.
- universal indicator paper (cut into 1 cm strips)
- white tile or sheet of white printer paper
- glass rod or plastic straw
- test tube or small glass beaker
- plastic syringe (2.5 ml capacity)
- hydrochloric acid (HCl) solution (0.1 M)
Instructions for preparation are given with the previous investigation: The reaction between magnesium oxide and hydrochloric acid
METHOD:
- Prepare the universal indicator paper by neatly placing five 1 cm pieces in a column on the white tile or sheet of printer paper.
-
Use the syringe to transfer 2 ml of the sodium hydroxide solution into the test tube or small glass beaker. We will be calling this the test solution from now on.
- Rinse the syringe very thoroughly with water and dry it out with a clean tissue. Now fill it with hydrochloric acid solution and set it aside.
- Transfer one drop of the sodium hydroxide (test solution) to the first piece of universal indicator paper.
- Compare the colour of the paper with the colour guide to find the pH of the sodium hydroxide solution. Record this pH in your results table.
- Add 0.5 ml of the hydrochloric acid solution from the syringe to the test solution. Stir it gently with the glass rod or straw and transfer another drop of the test solution to a fresh strip of the universal indicator paper.
- Read the pH of the solution off the colour guide and record it in your results table.
Learner dependent answer. Should be around 2 ml.
- If you are quite sure that all the base has been neutralised by the acid (the pH should be 7 and the universal indicator paper should have turned green), pour the test solution into a small glass beaker and leave it in the window sill for a few days. Remember to come back to it later to see what has happened to it.
NaCl forms in this reaction and the idea is for learners to let it dry out in the window sill and examine it later. It is probably not a good idea to let them taste it, as there is a possibility that not all of the acid or base has been neutralised.
RESULTS:
learner-dependent answer
-
Draw a graph of your results.
What is your independent variable? (Which variable did you change?)
Volume of HCl
What is your dependent variable? (Which variable did you measure?)
pH
The volume of HCl added should be on the x-axis of the graph and pH should be on the y-axis. There should be a general trend downwards (since acid is added to a base, we can expect the pH to drop), but it should not be linear. See comments and graph provided with the previous investigation.
CONCLUSIONS:
What conclusions can be made from the results of your investigation? Here you can rewrite your hypothesis, but change it to reflect your findings if they are different from what you predicted earlier.
Were you able to confirm or reject your hypothesis?
Now that we have investigated a reaction between a metal hydroxide (NaOH) and an acid (HCl), we can write an equation for the reaction. We will begin by writing a general equation and end with one that matches the reaction that we have just investigated.
A video illustrating the reaction of magnesium hydroxide with hydrochloric acid in the presence of universal indicator
General equation for the reaction of an acid with a metal hydroxide
You learnt that an acid-base reaction can be represented by the following general word equation:
acid + base → salt + water
The base in our reaction was a metal hydroxide, so the general equation becomes:
acid + metal hydroxide → salt + water
This is the general equation for the reaction between an acid and a metal hydroxide. The type of salt that forms will depend on the specific acid and metal hydroxide which were used in the reaction.
Equations for the reaction between sodium hydroxide and hydrochloric acid
Now we are going to learn how to write equations for our actual reaction.
Writing the chemical equation
The following steps will guide you:
HCl
Sodium hydroxide (NaOH)
Now,let's try to predict the products of the reaction. We know that water will be one of the products.
Na
Write what remains of the acid after we have taken away the H to make water. Remember we need two H to make one H2O, but NaOH has already contributed one O and one H. Now put the two fragments together. Place the metal from the base first and the non-metal from the acid. One Na and one Cl makes...
NaCl
Now, let's put it all together, in the following order: Acid + metal hydroxide → salt + water
HCl + NaOH → NaCl + H2O
Let's check quickly if the reaction is balanced.
- How many H atoms on the left and on the right? Are they balanced?
- How many Cl atoms on the left and on the right? Are they balanced?
- How many O atoms on the left and on the right? Are they balanced?
-
2 H atoms on the left and 2 H atoms on the right. The H's are balanced.
-
1 Cl atoms on the left and 1 Cl atoms on the right. The Cl's are balanced.
-
1 O atoms on the left and 1 O atoms on the right. The O's are balanced.
Once you have performed this reaction and you are left with a neutral solution, you decide you want to recover the sodium chloride (table salt). How will you do this?
You need to evaporate the water so that the salt crystallizes, either by leaving it in a sunny spot or boiling the solution.
Finally, let's use the chemical equation to write a word equation for the reaction:
hydrochloric acid + sodium hydroxide → sodium chloride + water
In the next section we are going to look at the reactions between acids and metal carbonates.
The general reaction of an acid with a metal carbonate
- metal carbonate
In this section we will investigate the reaction between an acid and a metal carbonate.

The reaction between calcium carbonate (chalk) and hydrochloric acid
Grind up a few pieces of white chalk for this experiment. The calcium carbonate will not actually dissolve well in water, but it should be possible to determine that the solution is basic, from the tiny amount of calcium carbonate that will dissolve when the chalk dust is suspended in water.
Learners will need their colour charts and results tables before they start.
The purpose of this investigation is to:
- test whether calcium carbonate is acidic or basic;
- determine whether the reaction between calcium carbonate and hydrochloric acid is a neutralisation reaction; and
- determine the products of the reaction between calcium carbonate and hydrochloric acid.
INVESTIGATIVE QUESTIONS:
Some ideas:
- When calcium carbonate is dissolved in water, will the resulting solution be acidic, basic or neutral?
- When a solution of calcium carbonate is treated with hydrochloric acid, will the pH of the mixture increase, decrease or stay the same?
- Will it be possible to neutralise all the calcium carbonate by adding hydrochloric acid? (Be careful not to introduce misconceptions here)
- What other products will form when calcium carbonate reacts with hydrochloric acid?
OVERVIEW OF THE INVESTIGATION :
We will measure the pH of a suspension of calcium carbonate (CaCO3) with universal indicator paper to confirm whether it is acidic or basic. Within what range do you expect the pH of the calcium carbonate to fall?
In the range pH > 7
The pH will decrease
HYPOTHESIS:
Some ideas:
- Calcium carbonate solution will have a pH greater than 7
- When calcium carbonate is treated with hydrochloric acid, the pH of the mixture will decrease
- By adding hydrochloric acid to the calcium carbonate, it should be able to decrease the pH to 7 and even below 7
MATERIALS:
- chalk dust (calcium carbonate) suspended in a small quantity of water.
- universal indicator paper (cut into 1 cm strips)
- white tile or sheet of white printer paper
- glass rod or plastic straw
- test tube or small glass beaker
- plastic syringe (2.5 cm capacity) or dropper
- hydrochloric acid (HCl) solution (0.1 M)
METHOD:
- Prepare the universal indicator paper by neatly placing five 1 cm pieces in a column on the white tile or sheet of printer paper.
-
Place approximately 2 ml of the calcium carbonate suspension into the test tube or small glass beaker. We will be calling this the test solution from now on.
- Rinse the syringe very thoroughly with water and dry it out with a clean tissue. Now fill it with hydrochloric acid solution and set it aside.
- Transfer one drop of the calcium carbonate (test solution) to the first piece of universal indicator paper.
- Compare the colour of the paper with the colour guide below, to find the pH of the calcium carbonate solution. Record this pH in your results table.
- Add 0.5 cm of the hydrochloric acid solution from the syringe to the test solution. Watch very carefully what happens. Do you see anything interesting? (Hint: Look for bubbles!) Stir the test solution gently with the glass rod and transfer another drop of it to a fresh strip of the universal indicator.
- Read the pH of the solution off the colour guide and record it in your table.
- Repeat steps 6 and 7 until the pH of the test solution reaches approximately 7. How much of the hydrochloric acid solution have you used? Write the volume in the space below.
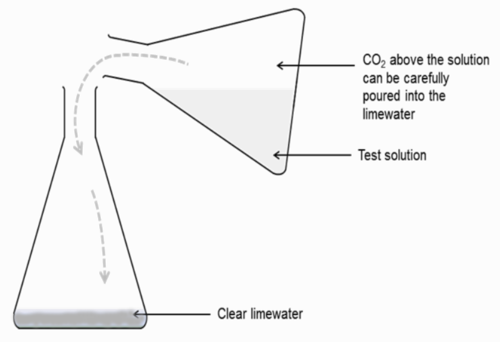
- Your teacher will repeat the experiment as a demonstration and will collect the gas that formed during the reaction, for testing with clear limewater.
Perform the experiment in a conical flask, as follows:
-
2 into another conical flask containing lime water (see diagram below). The CO2 gas should be poured out. Shake the conical flask containing the lime water and CO2 to facilitate mixing. Allow the learners to make their observations.2 is denser than air and will remain in the conical flask for a few minutes before diffusing into the air. It is during this time that you should pour it over into the lime water. Be careful not to let any of the test solution flow over into the clear lime water. Only the CO
Alternatively, you could use a setup like the one shown in the diagram below:
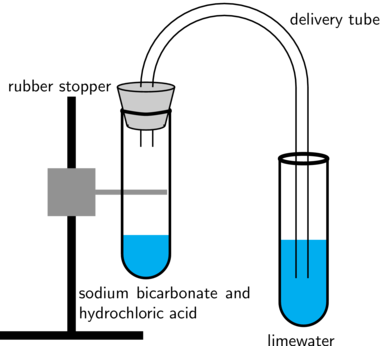
These experiments can also be done using combo plates.
Carbon dioxide, CO2
RESULTS:
- Present your results in a neat table. Use appropriate headings for your table. 'Volume of HCl added' and 'Colour of the universal indicator paper' and 'pH of the test solution' are suggested headings for your columns.
-
Draw a graph of your results.
What is your independent variable? (Which variable did you change?)
Volume of HCl
What is your dependent variable? (Which variable did you measure?)
pH
CONCLUSIONS:
What conclusions can be made from the results of your investigation? Here you can rewrite your hypothesis, but change it to reflect your findings if they are different from what you predicted earlier.
Were you able to confirm or reject your hypothesis?
Now that we have investigated a reaction between a metal carbonate (CaCO3) and an acid (HCl), we can write an equation for the reaction. We will begin by writing a general equation and end with one that matches the reaction that we have just investigated.
A video showing the reaction between metal carbonates and acids
General equation for the reaction of an acid with a metal carbonate
The general equation for the reaction between an acid and a base is as follows:
acid + base → salt + water
If we replace 'base' with 'metal carbonate', we get:
acid + metal carbonate → salt + water
But wait, there was a third product in our reaction! Can you remember what it was? (Hint: Bubbles formed, so it was a gas.)
CO2
We need to make it clear that CO2 was a product of the reaction, so the correct general word equation would be:
acid + metal carbonate → salt + water + carbon dioxide
The type of salt that forms will depend on the specific acid and metal carbonate which were used in the reaction.
Equations for the reaction between calcium carbonate and hydrochloric acid
Now we are going to learn how to write equations for our actual reaction.
Writing the chemical equation
The following steps will guide you:
HCl
Calcium carbonate (CaCO3)
Write what remains of the base after we have taken away the CO3 to make CO2 and leave one O to make water.
Ca
Write what remains of the acid after we have taken away the H to make water. Remember we need two H to make one H2O and CaCO3 has only contributed one O.
2 HCl are needed, so 2 Cl will remain.
CaCl2
Now, let's put it all together, first the reactants, then the products:
2 HCl + CaCO3→ CaCl2 + H2O + CO2
-
Let's check quickly if the reaction is balanced.
How many H on the left and right? Are they balanced?
2 H left and 2 H right. The H's are balanced.
How many Cl on the left and right? Are they balanced?
2 Cl left and 2 Cl right. The Cl's are balanced.
How many O on the left and right? Are they balanced?
3 O left and 3 O right. The O's are balanced.
How many C on the left and right? Are they balanced?
1 C left and 1 C right. The C's are balanced.
Finally, let's use the chemical equation to write a word equation for the reaction:
hydrochloric acid + calcium carbonate → calcium chloride + water + carbon dioxide
Applications for calcium carbonate
Calcium carbonate is found in many places outside of the laboratory. It is found in different types of rocks around the world, for example limestone, chalk and marble.

Calcium carbonate is also the main part of shells of various marine organisms, snails, pearls, oysters and bird eggshells. It is also found in the exoskeletons of crustaceans (such as crabs, prawns and lobsters).

Dark green leafy vegetables such as broccoli, kale and cabbage are a dietary source of calcium carbonate, providing the body with calcium. You can also take calcium carbonate in the form of tablet supplements.
Calcium carbonate also has many applications. In industry, the main application is in construction as it is used in various building materials and in cement. Calcium carbonate is used in many adhesives, paints and in ceramics. It is also used in swimming pools to adjust the pH. When do you think it would be added? If the pool was too acidic and you wanted to make it more basic, or if the pool was too basic and you wanted to make it more acidic?
CaCO3 forms a basic solution in water so it is used if the pH is too low (too acidic) and you want to make the pool water more basic.
Calcium carbonate is also used in agriculture in the form of lime powder. Agricultural lime is made by grinding up limestone or chalk. It is added to the soil if the soil is too acidic to increase the pH. It also provides plants with a source of calcium.

In this chapter we have investigated a number of reactions of acids with bases. We have learnt to write word equations for these reactions and practised converting between word and balanced chemical equations.
Summary
- The reaction of an acid with a base is called a neutralisation reaction.
- When an acid (pH 7), the pH of the resulting mixture will lie somewhere between that of the acid and the base. Even though the acid and base will be neutralised, the resulting solution will not necessarily be neutral.
-
Some common laboratory acides are sulfuric acid (H2SO4), nitric acid (HNO3) and hydrochloric acid (HCl).
- Non-metal oxides tend to form acidic solutions when they dissolve in water. These solutions will have pH values below 7.
- Metal oxides, metal hydroxides and metal carbonates form basic solutions in water; these will have pH values above 7.
- When a metal oxide, or a metal hydroxide reacts with an acid, a salt and water form as products.
- When a metal carbonate reacts with an acid, a salt, water and carbon dioxide form as products.
- The general word equations for the reactions of this chapter are the following:
-
acid + metal oxide → salt + water
-
acid + metal hydroxide → salt + water
-
acid + metal carbonate → salt + water + carbon dioxide
-
Concept map
Complete the concept map by filling in the blank spaces..
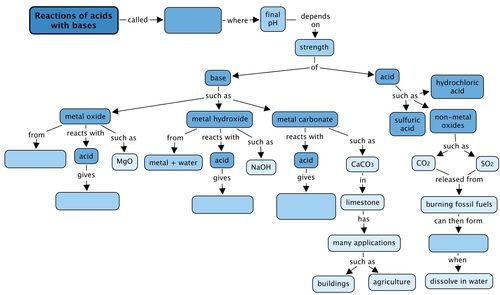
This is the completed concept map.
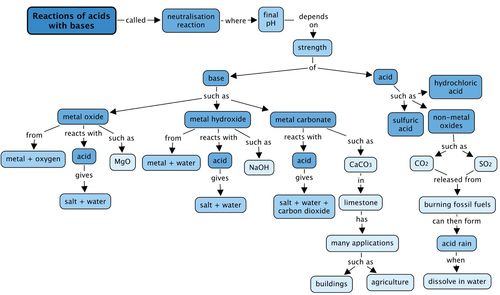
Revision Questions
-
Fill in the missing words in these sentences. Write the word on the line below. [10 marks]
To know if something is an acid or a base, we measure its _______.
pH
The name of the laboratory acid with the formula H2SO4, is _______.
sulfuric acid
The formula of the laboratory acid named hydrochloric acid, is _______.
HCl
When a metal oxide reacts with an ______, a salt and water will be formed.
acid
When a metal hydroxide reacts with an acid, a salt and _____ will be formed.
water
When a metal carbonate reacts with an acid, a salt, water and _______ will be formed.
carbon dioxide
Metal oxides, metal hydroxides and metal carbonates all dissolve in water, forming ______ solutions. This means the solutions will have pH values ____ than 7.
basic; greater
The reaction of an acid with a base is called a _______ reaction.
neutralisation
Non-metal oxides tend to form ______ solutions when they dissolve in water.
acidic
-
Write a short paragraph (3 or more sentences) to explain what you understand each of the following terms to mean, in your own words. [2 x 3 = 6 marks]
neutralisation
Learner's paragraph should contain at least the following ideas:
- When an acid and a base are mixed, the acid will lose some of its 'acidity' and the base will lose some of its 'basicity'.
-
If they are mixed in the right amounts, they will neutraliseeach other.
- The products of the reaction will be a salt and water.
acid rain
Learner's paragraph should contain at least the following ideas:
- Certain industries (and even some natural phenomena like volcanic eruptions) produce non-metal oxides as waste products.
- Non-metal oxides form acidic solutions when they dissolve in atmospheric water droplets.
- These acidic solutions rain down onto the Earth's surface and can cause damage to buildings, plant life and acidify water sources.
-
For each of the following reactions, complete the tables by providing the missing equations.
The reaction between hydrochloric acid and magnesium oxide [4 marks]
Word equation
Chemical equation
General equation
acid + metal oxide → salt + water
Word equation
hydrochloric acid + magnesium oxide → magnesium chloride + water
Chemical equation
2 HCl + MgO → MgCl2 + H2O
General equation
acid + metal oxide → salt + water
The reaction between hydrochloric acid and sodium hydroxide [6 marks]
Word equation
Chemical equation
General equation
Word equation
hydrochloric acid + sodium hydroxide → sodium chloride + water
Chemical equation
HCl + NaOH → NaCl + H2O
General equation
acid + metal hydroxide → salt + water
The reaction between hydrochloric acid and calcium carbonate [4 marks]
Word equation
Chemical equation
2 HCl + CaCO3 → CaCl2 + H2O + CO2
General equation
Word equation
hydrochloric acid + calcium carbonate → calcium chloride + water + carbon dioxide
Chemical equation
2 HCl + CaCO3 → CaCl2 + H2O + CO2
General equation
acid + metal carbonate → salt + water + carbon dioxide
The reaction between hydrochloric acid and magnesium hydroxide [4 marks]
Word equation
Chemical equation
2 HCl + Mg(OH)2 → MgCl2 + 2 H2O
General equation
Word equation
hydrochloric acid + magnesium hydroxide → magnesium chloride + water
Chemical equation
2 HCl + Mg(OH)2 → MgCl2 + 2 H2O
General equation
acid + metal hydroxide → salt + water
The reaction between hydrochloric acid and calcium oxide [4 marks]
Word equation
Chemical equation
2 HCl + CaO → CaCl2 + H2O
General equation
Word equation
hydrochloric acid + calcium oxide → calcium chloride + water
Chemical equation
2 HCl + CaO → CaCl2 + H2O
General equation
acid + metal oxide → salt + water
The reaction between hydrochloric acid and potassium hydroxide [6 marks]
Word equation
Chemical equation
General equation
Word equation
hydrochloric acid + potassium hydroxide → potassium chloride + water
Chemical equation
HCl + KOH → KCl + H2O
General equation
acid + metal hydroxide → salt + water
The reaction between hydrochloric acid and sodium carbonate [4 marks]Word equation
hydrochloric acid + sodium carbonate → sodium chloride + water + carbon dioxide
Chemical equation
General equation
Word equation
hydrochloric acid + sodium carbonate → sodium chloride + water + carbon dioxide
Chemical equation
2 HCl + Na2CO3 → 2 NaCl + H2O + CO2
General equation
acid + metal carbonate → salt + water + carbon dioxide
Total [48 marks]
