Explain the difference between the valence electrons and the valency of an element.
6.3 Covalent bonding
|
Previous
6.2 Lewis structures
|
Next
6.4 Ionic bonding
|
6.3 Covalent bonding (ESABT)
The nature of the covalent bond (ESABU)
Covalent bonding occurs between the atoms of non-metals. The outermost orbitals of the atoms overlap so that unpaired electrons in each of the bonding atoms can be shared. By overlapping orbitals, the outer energy shells of all the bonding atoms are filled. The shared electrons move in the orbitals around both atoms. As they move, there is an attraction between these negatively charged electrons and the positively charged nuclei. This attractive force holds the atoms together in a covalent bond.
- Covalent bond
-
Covalent bonding is a form of chemical bonding where pairs of electrons are shared between atoms.
You will have noticed in Table 6.1 that the number of electrons that are involved in bonding varies between atoms.
There is a relationship between the valency of an element and its position on the periodic table. For the elements in groups 1 and 2, the valency is the group number. For the elements in groups 13–18, the valency is the group number minus 10. For the transition metals, the valency can vary. In these cases we indicate the valency by a roman numeral after the element name, e.g. iron (III) chloride.
We can say the following:
-
A single covalent bond is formed when two electrons are shared between the same two atoms, one electron from each atom.
-
A double covalent bond is formed when four electrons are shared between the same two atoms, two electrons from each atom.
-
A triple covalent bond is formed when six electrons are shared between the same two atoms, three electrons from each atom.
You should also have noticed that compounds can have a mixture of single, double and triple bonds and that an atom can have several bonds. In other words, an atom does not need to share all its valence electrons with one other atom, but can share its valence electrons with several different atoms.
We say that the valency of the atoms is different.
- Valency
-
The number of electrons in the outer shell of an atom which are able to be used to form bonds with other atoms.
Below are a few examples. Remember that it is only the valence electrons that are involved in bonding and so when diagrams are drawn to show what is happening during bonding, it is only these electrons that are shown.
Worked example 1: Covalent bonding
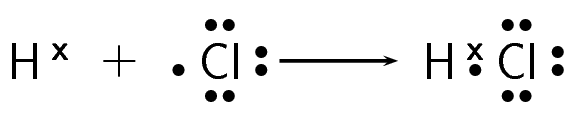
How do hydrogen and chlorine atoms bond covalently in a molecule of hydrogen chloride?
Determine the electron configuration of each of the bonding atoms
A chlorine atom has 17 electrons and an electron configuration of \([\text{Ne}]3\text{s}^{2}3\text{p}^{5}\). A hydrogen atom has only one electron and an electron configuration of \(1\text{s}^{1}\).
Determine how many of the electrons are paired or unpaired
Chlorine has seven valence electrons. One of these electrons is unpaired. Hydrogen has one valence electron and it is unpaired.
Work out how the electrons are shared
The hydrogen atom needs one more electron to complete its outermost energy level. The chlorine atom also needs one more electron to complete its outermost energy level. Therefore one pair of electrons must be shared between the two atoms. A single covalent bond will be formed.
Worked example 2: Covalent bonding involving multiple bonds
How do nitrogen and hydrogen atoms bond to form a molecule of ammonia \((\text{NH}_{3})\)?
Give the electron configuration
A nitrogen atom has seven electrons, and an electron configuration of \([\text{He}]2\text{s}^{2}2\text{p}^{3}\). A hydrogen atom has only one electron, and an electron configuration of \(1\text{s}^{1}\).
Give the number of valence electrons
Nitrogen has five valence electrons. Three of these electrons are unpaired. Hydrogen has one valence electron and it is unpaired.
Work out how the electrons are shared
Each hydrogen atom needs one more electron to complete its valence energy shell. The nitrogen atom needs three more electrons to complete its valence energy shell. Therefore three pairs of electrons must be shared between the four atoms involved. Three single covalent bonds will be formed.
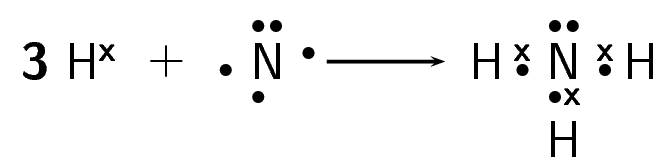
Worked example 3: Covalent bonding involving a double bond
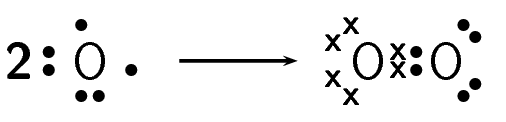
How do oxygen atoms bond covalently to form an oxygen molecule?
Determine the electron configuration of the bonding atoms.
Each oxygen atom has eight electrons, and their electron configuration is \([\text{He}]2\text{s}^{2}2\text{p}^{4}\).
Determine the number of valence electrons for each atom and how many of these electrons are paired and unpaired.
Each oxygen atom has six valence electrons. Each atom has two unpaired electrons.
Work out how the electrons are shared
Each oxygen atom needs two more electrons to complete its valence energy shell. Therefore two pairs of electrons must be shared between the two oxygen atoms so that both outermost energy levels are full. A double bond is formed.
Properties of covalent compounds (ESABV)
Covalent compounds have several properties that distinguish them from ionic compounds and metals. These properties are:
-
The melting and boiling points of covalent compounds are generally lower than those of ionic compounds.
-
Covalent compounds are generally more flexible than ionic compounds. The molecules in covalent compounds are able to move around to some extent and can sometimes slide over each other (as is the case with graphite, which is why the lead in your pencil feels slightly slippery). In ionic compounds, all the ions are tightly held in place.
-
Covalent compounds generally are not very soluble in water, for example plastics are covalent compounds and many plastics are water resistant.
-
Covalent compounds generally do not conduct electricity when dissolved in water, for example iodine dissolved in pure water does not conduct electricity.
Covalent bonding
Complete the table below by filling in the number of valence electrons for each of the elements shown:
| Element | Group number | No. of valence electrons | No. of electrons needed to fill outer shell |
| \(\text{He}\) | |||
| \(\text{Li}\) | |||
| \(\text{B}\) | |||
| \(\text{C}\) | |||
| \(\text{F}\) | |||
| \(\text{Ne}\) | |||
| \(\text{Na}\) | |||
| \(\text{Al}\) | |||
| \(\text{P}\) | |||
| \(\text{S}\) | |||
| \(\text{Ca}\) | |||
| \(\text{Kr}\) |
Draw simple diagrams to show how electrons are arranged in the following covalent molecules:
- hydrogen sulphide \((\text{H}_{2}\text{S})\)
- chlorine \((\text{Cl}_{2})\)
- nitrogen \((\text{NH}_{2})\)
- carbon monoxide \((\text{CO})\)
|
Previous
6.2 Lewis structures
|
Table of Contents |
Next
6.4 Ionic bonding
|
