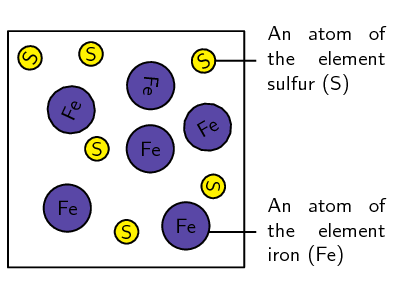
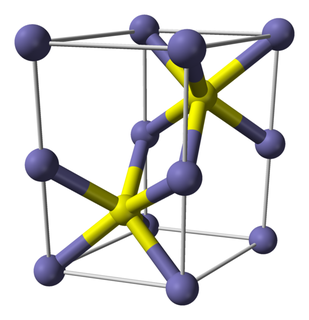
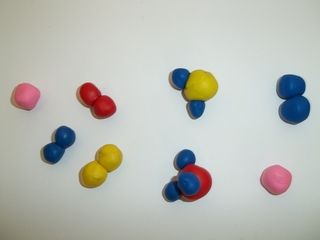In the following table, tick whether each of the substances listed is a mixture or a pure substance. If it is a mixture, also say whether it is a homogeneous or heterogeneous mixture.
|
Substance |
Mixture or pure |
Homogeneous or heterogeneous mixture |
|
fizzy colddrink |
||
|
steel |
||
|
oxygen |
||
|
iron filings |
||
|
smoke |
||
|
limestone (\(\text{CaCO}_{3}\)) |