4.1 Introduction
|
Previous
End of chapter exercises
|
Next
4.2 Models of the atom
|
Chapter 4: The atom
4.1 Introduction (ESAAM)
We have now looked at many examples of the types of matter and materials that exist around us and we have investigated some of the ways that materials are classified. But what is it that makes up these materials? And what makes one material different from another? In order to understand this, we need to take a closer look at the building blocks of matter — the atom. Atoms are the basis of all the structures and organisms in the universe. The planets, sun, grass, trees, air we breathe and people are all made up of different combinations of atoms.
Library assignment: Models of the atom
Our current understanding of the atom came about over a long period of time, with many different people playing a role. Conduct some research into the development of at least five different ideas of the atom and the people who contributed to it.
Some suggested people to look at are: JJ Thomson, Ernest Rutherford, Marie Curie, JC Maxwell, Max Planck, Albert Einstein, Niels Bohr, Lucretius, LV de Broglie, CJ Davisson, LH Germer, Chadwick, Werner Heisenberg, Max Born, Erwin Schrodinger, John Dalton, Empedocles, Leucippus, Democritus, Epicurus, Zosimos, Maria the Jewess, Geber, Rhazes, Robert Boyle, Henry Cavendish, A Lavoisier and H Becquerel. You do not need to find information on all these people, but try to find information about as many of them as possible. Make a list of five key contributions to a model of the atom and then make a timeline of this information. (You can use an online tool such as Dipity (www.dipity.com) to make a timeline.) Try to get a feel for how it all eventually fit together into the modern understanding of the atom.
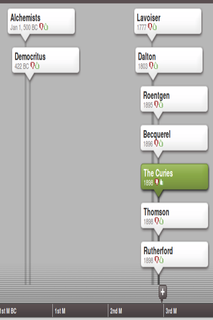
Figure 4.1: A timeline of atomic theory.
|
Previous
End of chapter exercises
|
Table of Contents |
Next
4.2 Models of the atom
|
