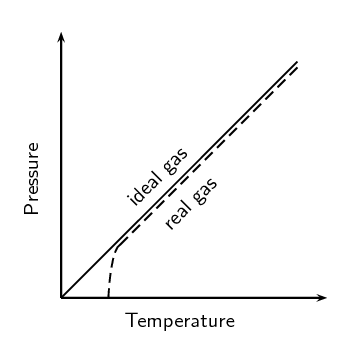
Summarise the difference between a real gas and an ideal gas in the following table:
| Property | Ideal gas | Real gas |
| Size of particles | ||
| Attractive forces | ||
| Speed of molecules |
| Property | Ideal gas | Real gas |
| Size of particles | No volume and can be ignored | Non-negligible volume |
| Attractive forces | No attractive forces | Attractive forces |
| Speed of molecules | All molecules at the same speed. | Molecules move at different speeds and we use the average speed. |